Satellite images reveal ocean acidification from space
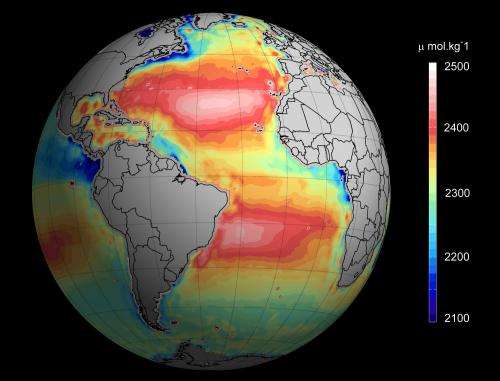
Pioneering techniques that use satellites to monitor ocean acidification are set to revolutionise the way that marine biologists and climate scientists study the ocean. This new approach, that will be published on the 17 February 2015 in the journal Environmental Science and Technology, offers remote monitoring of large swathes of inaccessible ocean from satellites that orbit the Earth some 700 km above our heads.
Each year more than a quarter of global CO2 emissions from burning fossil fuels and cement production are taken up by the Earth's oceans. This process turns the seawater more acidic, making it more difficult for some marine life to live. Rising CO2 emissions, and the increasing acidity of seawater over the next century, has the potential to devastate some marine ecosystems, a food resource on which we rely, and so careful monitoring of changes in ocean acidity is crucial.
Researchers at the University of Exeter, Plymouth Marine Laboratory, Institut français de recherche pour l'exploitation de la mer (Ifremer), the European Space Agency and a team of international collaborators are developing new methods that allow them to monitor the acidity of the oceans from space.
Dr Jamie Shutler from the University of Exeter who is leading the research said: "Satellites are likely to become increasingly important for the monitoring of ocean acidification, especially in remote and often dangerous waters like the Arctic. It can be both difficult and expensive to take year-round direct measurements in such inaccessible locations. We are pioneering these techniques so that we can monitor large areas of the Earth's oceans allowing us to quickly and easily identify those areas most at risk from the increasing acidification."
Current methods of measuring temperature and salinity to determine acidity are restricted to in situ instruments and measurements taken from research vessels. This approach limits the sampling to small areas of the ocean, as research vessels are very expensive to run and operate.
The new techniques use satellite mounted thermal cameras to measure ocean temperature while microwave sensors measure the salinity. Together these measurements can be used to assess ocean acidification more quickly and over much larger areas than has been possible before.
Dr Peter Land from Plymouth Marine Laboratory who is lead author of the paper said: "In recent years, great advances have been made in the global provision of satellite and in situ data. It is now time to evaluate how to make the most of these new data sources to help us monitor ocean acidification, and to establish where satellite data can make the best contribution."
A number of existing satellites can be used for the task; these include the European Space Agency's Soil Moisture and Ocean Salinity (SMOS) sensor that was launched in 2009 and NASA's Aquarius satellite that was launched in 2011.
The development of the technology and the importance of monitoring ocean acidification are likely to support the development of further satellite sensors in the coming years.
More information: The research is published in the scientific journal Environmental Science and Technology: pubs.acs.org/doi/abs/10.1021/es504849s
Journal information: Environmental Science and Technology
Provided by University of Exeter




















