Stressed out: Research sheds new light on why rechargeable batteries fail
Pity the poor lithium ion. Drawn relentlessly by its electrical charge, it surges from anode to cathode and back again, shouldering its way through an elaborate molecular obstacle course. This journey is essential to powering everything from cell phones to cordless power tools. Yet, no one really understands what goes on at the atomic scale as lithium ion batteries are used and recharged, over and over again.
Michigan Technological University researcher Reza Shahbazian-Yassar has made it his business to better map the ion's long, strange trip—and perhaps make it smoother and easier. His ultimate aim: to make better batteries, with more power and a longer life.
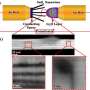
Using transmission electron microscopy, Anmin Nie, a senior postdoctoral researcher in Shahbazian-Yassar's research group, has recently documented what can happen to anodes as lithium ions work their way into them, and it's not especially good. The research was recently published in the journal Nano Letters.
"We call it atomic shuffling," says Shahbazian-Yassar, the Richard and Elizabeth Henes Associate Professor in Nanotechnology. "The layered structure of the electrode changes as the lithium goes inside, creating a sandwich structure: there is lots of localized expansion and contraction in the electrode crystals, which helps the lithium blaze a trail through the electrode."
The atomic shuffling not only helps explain how lithium ions move through the anode, in this case a promising new material called zinc antimonide. It also provides a clue as to why most anodes made of layered materials eventually fail. "We showed that the ions cause a lot of local stress and phase transitions," Anmin said.
More information: "Lithiation-Induced Shuffling of Atomic Stacks," Nano Letters, 2014.
Journal information: Nano Letters
Provided by Michigan Technological University