Blocking African sleeping sickness' tiny culprit
A tsetse fly bites a girl. She becomes itchy, feverish, and her joints ache. Weeks later, she loses coordination and some sensation in her limbs. It becomes difficult to think, to sleep.
This is what it feels like when you have African trypanosomiasis, better known as sleeping sickness.
Infected tsetse flies carry the culprit parasites: Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Sleeping sickness affected about 7,000 people in 2012, but it could be poised for an epidemic, if, as in the 1960s, strict controls are not maintained.
Using the Canadian Light Source synchrotron, a team of researchers in Switzerland, Germany and Canada recently published a paper suggesting an improved way to target and treat sleeping sickness while causing minimal harm to human hosts. The team's new potential treatment relies on a fundamental difference between Trypanosoma parasites and human cells in one simple cell function.
Dr. Emil Pai, professor in the departments of Biochemistry, Medical Biophysics, and Molecular Genetics at the University of Toronto, explained that most of the world uses glutathione reductase (GR) and its substrate glutathione, to keep the cellular reduction/oxidation levels constant. Trypanosoma, which cause sleeping sickness and a small handful of other conditions, use trypanothione reductase (TR) and trypanothione instead. Due to the core functions of both enzymes, if it is possible to block TR but not human GR, one could target the sleeping sickness carriers exclusively.
When Pai came onboard the project, Elke Persch and Dr. François Diederich of the Eidgenössische Technische Hochschule (ETH) in Zurich, Switzerland, together with several Swiss colleagues, and Dr. Luise Krauth-Siegel of the University of Heidelberg, Germany, had been making progress synthesizing and characterizing possible TR inhibitors, but they had encountered major problems in visualizing the site of and interaction between the inhibitor and enzyme target. That information would allow them to make changes of the molecular structure to improve the effectiveness of the inhibitors. At that point Diederich and Pai ran into one another at an airport.
Krauth-Siegel, Diederich, and Pai knew each other well from their time as graduate students at the Max-Planck Institute for Medical Research and the University of Heidelberg. Pai offered to help with crystallizing the enzyme–inhibitor complexes for study.
"Luise (Krauth-Siegel) is a real hard core biochemist, Francois (Diederich) is a synthetic and physical organic chemist and I'm kind of a little bit of everything. Those personal connections can lead to very exciting science," Pai said.
Elke Persch in Diederich's laboratory had worked for some time synthesizing smaller TR inhibitors. Dr. Steve Bryson in Pai's group was successful in generating workable crystals, and sent the samples to the CLS CMCF crystallography beamline.
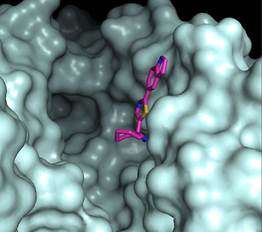
Armed with these crystal samples, and the CLS's X-ray imaging capabilities, the researchers were able to observe in detail how the inhibitor and TR interact.
"What's a little bit remarkable about this paper is that a relatively small compound binds to a rather large active binding site on the TR enzyme, and still you get very specific binding to part of the active site," Pai said. "That's not trivial."
Like a keyhole, smaller, highly-defined binding sites are generally easiest to bind with by eliminating the risk of water or other compounds getting into the site and interfering.
To their surprise, the researchers found that the two very similar active sites of TR from T. brucei and T. cruzi, which causes Chagas' disease, mostly found in South America, bound the inhibitors in different orientations. That difference opens the door to the design of species-specific inhibitors.
After some further modifications, the team will likely hand the reigns in this research over to industry, while the researchers plan to explore other pure science problems.
Pai, for his part, has always worked this way. Despite having investigated problems related to diseases including malaria, gout, and HIV, he has always been drawn to his subjects by the fundamental concepts behind them.
"We've rarely started a project with a disease in mind. We've always chosen projects because of the science," he said. The results, though, are important real-world solutions.
More information: Persch, Elke, et al. "Binding to Large Enzyme Pockets: Small‐Molecule Inhibitors of Trypanothione Reductase." ChemMedChem (2014). DOI: 10.1002/cmdc.201402032
Journal information: ChemMedChem
Provided by Canadian Light Source

















