August 22, 2014 feature
Scientists fabricate defect-free graphene, set record reversible capacity for Co3O4 anode in Li-ion batteries
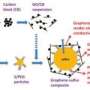
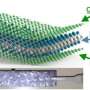
(Phys.org) —Graphene has already been demonstrated to be useful in Li-ion batteries, despite the fact that the graphene used often contains defects. Large-scale fabrication of graphene that is chemically pure, structurally uniform, and size-tunable for battery applications has so far remained elusive. Now in a new study, scientists have developed a method to fabricate defect-free graphene (df-G) without any trace of structural damage. Wrapping a large sheet of negatively charged df-G around a positively charged Co3O4 creates a very promising anode for high-performance Li-ion batteries.
The research groups of Professor Junk-Ki Park and Professor Hee-Tak Kim from Korea Advanced Institute of Science and Technology (KAIST) and Professor Yong-Min Lee's research group from Hanbat National University, all in Daejeon, South Korea, have published their paper on the new fabrication method in a recent issue of Nano Letters.
As the researchers explain, current methods to fabricate high-quality graphene fall into two categories: mechanical approaches and chemical approaches. While mechanical cleavage provides high-quality graphene, its low yield makes it insufficient for large-scale production. Chemical approaches, on the other hand, can produce bulk quantities but can involve imperfections.
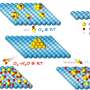
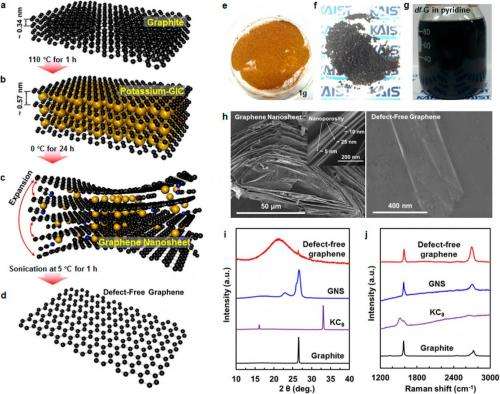
The new method differs from both of these types of methods and involves a few key steps. First the researchers filled a Pyrex tube with graphite powder, and then placed the open-ended tube inside a slightly larger tube. Then they added potassium to the bottom gap between the two tubes, sealed the tubes, and heated them. The heat causes the molten potassium to move inside the micropores between the graphite powders, so that the potassium molecules become intercalated into the graphite interlayers. The resulting potassium graphite compounds were then placed in a pyridine solution, which causes the layers to expand away from each other to form graphene nanosheets that could later be cooled and exfoliated one layer at a time.
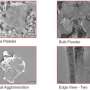
The researchers performed many sets of experiments in which they varied factors such as the temperatures and the type of solution, which are critical to control the quality and size of the df-G. They found that, by controlling the temperature of the exfoliation step, the size of the df-G can be varied between 0.25 to 14.0 µm2.
The researchers demonstrated that wrapping a large-sized negatively charged sheet of df-G around a positively charged piece of Co3O4 creates an anode with several impressive characteristics. Most significantly is its high capacity after many cycles (1050 mAh/g at 500 mA/g and 900 mAh/g at 1000 mAh/g even after 200 cycles). To the best of the researchers' knowledge, this reversible capacity is the highest among all Co3O4 electrodes ever reported.
The researchers explain that the large-sized df-G, with its perfect crystallinity, improves the anode performance because when a single graphene sheet is wrapped around a bundle of Co3O4 particles, the Co3O4 particles are prevented from becoming pulverized and then electrically detaching from the anode, which would otherwise occur. Because of this protective effect, the anode's capacity is preserved even after 200 cycles, whereas anodes with an imperfect graphene layer rapidly decrease with cycling. The large size of the graphene plays a key role in the performance because a larger size provides a higher cycling stability of the nanosized anode materials by improving their mechanical integrity.
With these advantages, the researchers expect the df-G to bring significant advances of composite electrodes for a variety of electrochemical system, including batteries, fuel cells, and capacitors.
More information: Kwang Hyun Park, et al. "Defect-Free, Size-Tunable Graphene for High-Performance Lithium Ion Battery." Nano Letters. DOI: 10.1021/nl500993q
Journal information: Nano Letters
© 2014 Phys.org