July 16, 2014 feature
Of catalysts and chirality: Highly-selective growth of structure-specific single-walled carbon nanotubes
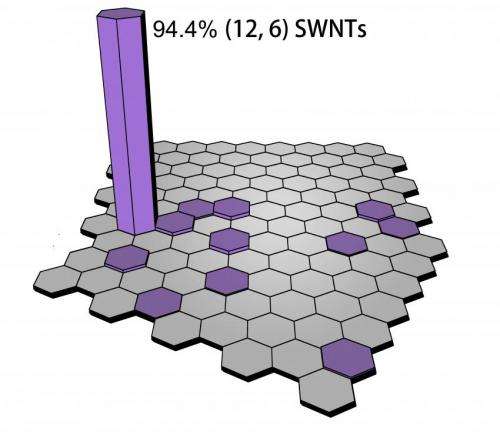
(Phys.org) —Carbon – the chemical basis of all known life and an element known as far back as the 8th century BC – exists in a range of forms, or allotropes, with remarkably diverse properties. (Diamond, for example, is transparent and extremely hard tetrahedral lattice that conducts electricity poorly but is an excellent thermal conductor. Graphite, on the other hand – a moderate electrical conductor – is a soft, black, flaky solid formed from sheets of flat hexagonal lattices known as graphene.) Among carbon's allotropes, carbon nanotubes are cylindrical graphene-based nanostructures with properties central to many fields of materials science and technology. In particular, single-walled carbon nanotubes (SWNTs) are carbon nanotubes whose properties change with their chirality – that is, the arrangements of the carbon atoms, which is based on tube diameter and wrapping angle as specified by what is known as their (n,m) value. These variants behave either as electrical conductors or semiconductors with different bandgaps (the energy range in a solid where no electron states can exist), making them extremely desirable for nanoelectronics applications. While this characteristic depends on the SWVTs all being in chiral form or the other, it has historically been very difficult to selectively grow one form alone, with the highest selectivity of 55% being achieved using carefully-chosen particles as catalysts in the chemical vapor deposition synthesis growth process. Recently, however, scientists at Peking University, Beijing have used tungsten-based bimetallic alloy nanocrystals as catalysts to directly produce single-chirality (that is, either left- or right-handed) SWNTs at a greater than 92% purity. By so doing, the researchers say, their results set the stage for complete control over SWNT chirality growth, and thereby further SWNT application development.
Prof. Yan Li discussed the paper she and her co-authors published in Nature with Phys.org. "The properties of SWNTs are totally determined by their structure, or chirality – and in many applications, it's required that materials present uniform properties," Li tells Phys.org. As an example, she says that when using SWNTs to build field effect transistors (FETs), it's always hoped that all SWNTs have the identical structure, thereby exhibiting the same performance. "However," Li adds, "chirality-controlled growth has been a great challenge in the field for twenty years – but we've developed a new strategy to realize the goal."
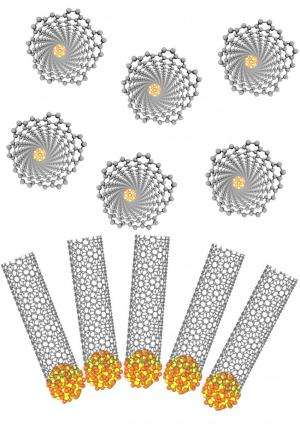
Li notes that there are two factors important for reducing the alloying temperature: tungsten and cobalt atoms being already well-mixed in the precursor, and the particles being of nanoscale dimensions. Accordingly, their strategy is based on a new family of catalysts – tungsten-based alloy nanocatalysts – for carbon nanotube growth. "These catalysts maintain their crystallized structure under the very high temperatures needed for carbon nanotube growth, and also exhibit a very unique structure that serves as a carbon nanotube template." The tungsten-based alloy forms at extremely high temperature – normally well above 2000°C –necessitating special facilities, since it is extremely difficult to perform this procedure using standard laboratory equipment – and in addition, Li points out, it is difficult to control the size, structure and morphology of the resultant alloy under such conditions. "We used a precursor molecular cluster† to obtain tungsten-cobalt (W–Co) alloy nanoparticle nanocatalysts at the moderate temperature of ~1000°C," Li says, "which made SWNT production much easier."
The key to solving this two decade-old chirality-controlled SWNT growth challenge was, stated simply, a new idea. "Though extensive effort has been made exploring chirality-selective SWNT growth, no efficient approach had been developed. This is partially due to our not having sufficient insight into the SWNT growth mechanism," she explains. "Indeed, it's quite difficult to collect enough information in situ during the nanotube growth process – but it's this very information that can help us to understand the mechanism. Fueled by my more than ten years' experience in SWNT growth, I had a novel idea about using catalysts to guide the structure of SWNTs."
While researchers have been vigorously investigating the use of catalysts to template SWNTs structure – as evidenced by the many papers published in this area – success has proven elusive. "We succeeded," Li adds, "because we have two significantly different ideas – namely, we recognized that catalysts with high melting points are necessary for using the catalyst as structural template; we found the right recipe to obtain catalysts with high melting points; we realized that the unique structure of the catalyst is essential to achieving high selectivity and specificity. Moreover, as inorganic chemists we've long known about molecular clusters, their characteristics and how to prepare them – so the idea using molecular clusters as the precursor for W-Co alloy nanoparticles came naturally to us, resulting in our designing the new pathway for preparing W-Co alloy nanoparticles."
In their paper, the scientists say that since using high-melting-point alloy nanocrystals with optimized structures as catalysts has demonstrably allowed production of single-chirality nanotubes at an abundance of >92%, they expect that their results will pave the way for total chirality control in SWNT growth, thereby promoting the development of SWNT applications. "Based-on our understanding about the SWNT growth mechanism and the experimental data we already have," Li says, "we're confident that our strategy of growing SWNTs with desired structure and chirality using catalysts with designed structure and high stability can become a standard approach." Moreover, tungsten, cobalt, iron, and nickel are abundant, inexpensive metals, and their carbon source is ethanol, so production costs can be low – an obvious advantage for future commercialization.
One of the most exciting potential applications is in electronics. Li points out that the 2009 International Technology Roadmap for Semiconductors (ITRS) selected carbon-based nanoelectronics – Including carbon nanotubes and graphene – as promising technologies targeting commercial demonstration in the next 10-15 year horizon, and so to receive additional resources and detailed road mapping. "For the large-scale application of SWNTs in nanoelectronics," Li points out, "SWNTs with identical structure are desired. Our method of growing SWNTs with identical structure is therefore a very important part of the development of carbon nanotube-based electronics."
Citing another example, Li notes that Prof. Lianmao Peng and his team have shown1 that SWNTs can be used to achieve efficient photovoltage multiplication in SWNT-based solar cells. She notes that structure-identical SWNTs can also be used in such devices, so if the nanotubes are used, solar cells with accurately adjusted photovoltage can be obtained. "There are definitely much more potential applications," Li adds. Now we have SWNT samples with identical structure, we can explore more interesting properties and possible applications that we could never before imagine."
Li also mentions their use of Vienna Ab-initio Simulation Package for self-consistent density functional theory simulations. "Simulation provides insights not readily available through experimental data alone. It can also help theorists understand the mechanism of various processes."
Moving forward, Li says, the scientists are focused on three key steps:
• Designing more catalysts to produce SWNTs with a wider range of chiralities
• Further optimizing the process to improve chirality selectivity, and therefore purity
• Exploring bulk synthesis
Beyond their own field, Li tells Phys.org, there are other areas of research that might benefit from their study. "In alloy metallurgy, our idea of using some special precursor to dramatically reduce the alloying temperature may be adopted because it may remarkably reduce energy consumption – and the lower-process temperature can greatly ease materials and control systems requirements for production apparatus. In addition, using alloy catalysts of unique structure to produce molecules with a predesigned structure can be widely used in chemical synthesis. Finally," Li concludes, "our methods for characterizing SWNT chirality composition can be used in basic carbon nanotube research."
More information: Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts, Nature 510, 522–524 (26 June 2014), doi:10.1038/nature13434
Related:
† Na15[Na3,{Co(H2O)4}6{WO(H2O)}3(P2W12O48)3]nH2O, denoted as {W39Co6Ox}
1Helicity-dependent single-walled carbon nanotube alignment on graphite for helical angle and handedness recognition, Nature Communications 4:2205 (29 July 2013), doi:10.1038/ncomms3205
Journal information: Nature , Nature Communications
© 2014 Phys.org