New catalyst converts carbon dioxide to fuel
Scientists from the University of Illinois at Chicago have synthesized a catalyst that improves their system for converting waste carbon dioxide into syngas, a precursor of gasoline and other energy-rich products, bringing the process closer to commercial viability.
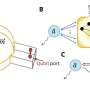
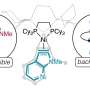
Amin Salehi-Khojin, UIC professor of mechanical and industrial engineering, and his coworkers developed a unique two-step catalytic process that uses molybdenum disulfide and an ionic liquid to "reduce," or transfer electrons, to carbon dioxide in a chemical reaction. The new catalyst improves efficiency and lowers cost by replacing expensive metals like gold or silver in the reduction reaction.
The study was published in the journal Nature Communications on July 30.
The discovery is a big step toward industrialization, said Mohammad Asadi, UIC graduate student and co-first author on the paper.
"With this catalyst, we can directly reduce carbon dioxide to syngas without the need for a secondary, expensive gasification process," he said. In other chemical-reduction systems, the only reaction product is carbon monoxide. The new catalyst produces syngas, a mixture of carbon monoxide plus hydrogen.
The high density of loosely bound, energetic d-electrons in molybdenum disulfide facilitates charge transfer, driving the reduction of the carbon dioxide, said Salehi-Khojin, principal investigator on the study.
"This is a very generous material," he said. "We are able to produce a very stable reaction that can go on for hours."
"In comparison with other two-dimensional materials like graphene, there is no need to play with the chemistry of molybdenum disulfide, or insert any host materials to get catalytic activity," said Bijandra Kumar, UIC post-doctoral fellow and co-first author of the paper.
"In noble metal catalysts like silver and gold, catalytic activity is determined by the crystal structure of the metal, but with molybdeneum disulfide, the catalytic activity is on the edges," said graduate student Amirhossein Behranginia, a coauthor on the paper. "Fine-tuning of the edge structures is relatively simple. We can easily grow the molybdenum disulfide with the edges vertically aligned to offer better catalytic performance."
The proportion of carbon monoxide to hydrogen in the syngas produced in the reaction can also be easily manipulated using the new catalyst, said Salehi-Khojin.
"Our whole purpose is to move from laboratory experiments to real-world applications," he said. "This is a real breakthrough that can take a waste gas—carbon dioxide—and use inexpensive catalysts to produce another source of energy at large-scale, while making a healthier environment."
More information: Nature Communications 30 Jul 2014 DOI: 10.1038/ncomms5470
Journal information: Nature Communications
Provided by University of Illinois at Chicago