New molecules around old stars
(Phys.org) —Using ESA's Herschel space observatory, astronomers have discovered that a molecule vital for creating water exists in the burning embers of dying Sun-like stars.
When low- to middleweight stars like our Sun approach the end of their lives, they eventually become dense, white dwarf stars. In doing so, they cast off their outer layers of dust and gas into space, creating a kaleidoscope of intricate patterns known as planetary nebulas.
These actually have nothing to do with planets, but were named in the late 18th century by astronomer William Herschel, because they appeared as fuzzy circular objects through his telescope, somewhat like the planets in our Solar System.
Over two centuries later, planetary nebulas studied with William Herschel's namesake, the Herschel space observatory, have yielded a surprising discovery.
Like the dramatic supernova explosions of weightier stars, the death cries of the stars responsible for planetary nebulas also enrich the local interstellar environment with elements from which the next generations of stars are born.
While supernovas are capable of forging the heaviest elements, planetary nebulas contain a large proportion of the lighter 'elements of life' such as carbon, nitrogen, and oxygen, made by nuclear fusion in the parent star.
A star like the Sun steadily burns hydrogen in its core for billions of years. But once the fuel begins to run out, the central star swells into a red giant, becoming unstable and shedding its outer layers to form a planetary nebula.
The remaining core of the star eventually becomes a hot white dwarf pouring out ultraviolet radiation into its surroundings.
This intense radiation may destroy molecules that had previously been ejected by the star and that are bound up in the clumps or rings of material seen in the periphery of planetary nebulas.
The harsh radiation was also assumed to restrict the formation of new molecules in those regions.
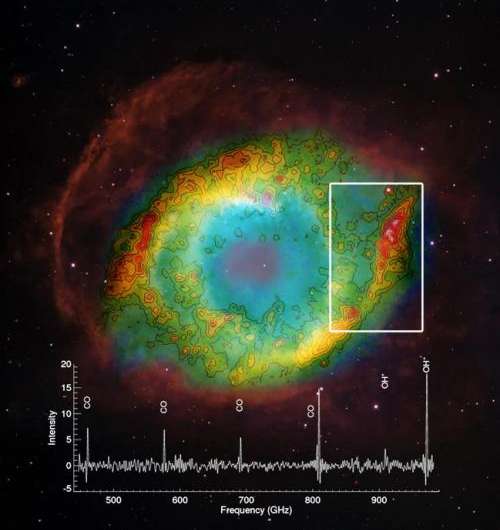
But in two separate studies using Herschel astronomers have discovered that a molecule vital to the formation of water seems to rather like this harsh environment, and perhaps even depends upon it to form. The molecule, known as OH+, is a positively charged combination of single oxygen and hydrogen atoms.
In one study, led by Dr Isabel Aleman of the University of Leiden, the Netherlands, 11 planetary nebulas were analysed and the molecule was found in just three.
What links the three is that they host the hottest stars, with temperatures exceeding 100 000ºC.
"We think that a critical clue is in the presence of the dense clumps of gas and dust, which are illuminated by UV and X-ray radiation emitted by the hot central star," says Dr Aleman.
"This high-energy radiation interacts with the clumps to trigger chemical reactions that leads to the formation of the molecules."
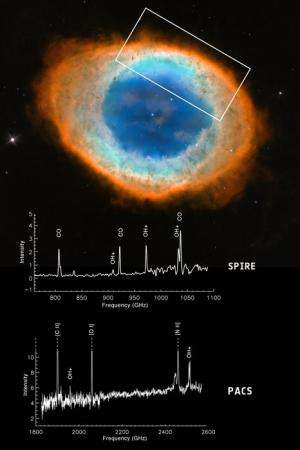
Meanwhile, another study, led by Dr Mireya Etxaluze of the Instituto de Ciencia de los Materiales de Madrid, Spain, focused on the Helix Nebula, one of the nearest planetary nebulas to our Solar System, at a distance of 700 light years.
The central star is about half the mass of our Sun, but has a far higher temperature of about 120 000ºC. The expelled shells of the star, which in optical images appear reminiscent of a human eye, are known to contain a rich variety of molecules.
Herschel mapped the presence of the crucial molecule across the Helix Nebula, and found it to be most abundant in locations where carbon monoxide molecules, previously ejected by the star, are most likely to be destroyed by the strong UV radiation.
Once oxygen atoms have been liberated from the carbon monoxide, they are available to make the oxygen–hydrogen molecules, further bolstering the hypothesis that the UV radiation may be promoting their creation.
The two studies are the first to identify in planetary nebulas this critical molecule needed for the formation of water, although it remains to be seen if the conditions would actually allow water formation to proceed.
"The proximity of the Helix Nebula means we have a natural laboratory on our cosmic doorstep to study in more detail the chemistry of these objects and their role in recycling molecules through the interstellar medium," says Dr Etxaluze.
"Herschel has traced water across the Universe, from star-forming clouds to the asteroid belt in our own Solar System," says Göran Pilbratt, ESA's Herschel project scientist.
"Now we have even found that stars like our Sun could contribute to the formation of water in the Universe, even as they are in their death throes."
More information: "Herschel planetary nebula survey (HerPlaNS). First detection of OH+ in planetary nebulae," by I. Aleman et al., and "Herschel spectral-mapping of the Helix Nebula (NGC 7293): extended CO photodissociation and OH+ emission," by M. Etxaluze et al., are published in Astronomy & Astrophysics.
Journal information: Astronomy & Astrophysics
Provided by European Space Agency



















