New method sneaks drugs into cancer cells before triggering release
(Phys.org) —Biomedical engineering researchers have developed an anti-cancer drug delivery method that essentially smuggles the drug into a cancer cell before triggering its release. The method can be likened to keeping a cancer-killing bomb and its detonator separate until they are inside a cancer cell, where they then combine to destroy the cell.
"This is an efficient, fast-acting way of delivering drugs to cancer cells and triggering cell death," says Dr. Ran Mo, lead author of a paper on the work and a postdoctoral researcher in the joint biomedical engineering program at North Carolina State University and the University of North Carolina at Chapel Hill. "We also used lipid-based nanocapsules that are already in use for clinical applications, making it closer to use in the real world."
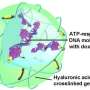
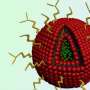
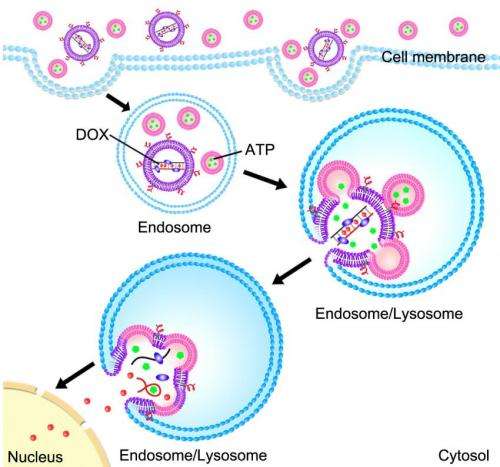
The technique uses nanoscale lipid-based capsules, or liposomes, to deliver both the drug and the release mechanism into cancer cells. One set of liposomes contains adenosine-5'-triphosphate (ATP), the so-called "energy molecule." A second set of liposomes contains an anti-cancer drug called doxorubicin (Dox) that is embedded in a complex of DNA molecules. When the DNA molecules come into contact with high levels of ATP, they unfold and release the Dox. The surface of the liposomes is integrated with positively charged lipids or peptides, which act as corkscrews to introduce the liposomes into cancer cells.
As the liposomes are absorbed into a cancer cell, they are sealed off from the rest of the cell in an endosome – a compartment that walls off all foreign material that gets into a cell.
The environment inside an endosome is acidic, which causes the Dox liposomes and ATP liposomes to fuse together, as well as to the wall of the endosome itself.
Meanwhile, two other things are happening simultaneously. First, the ATP liposomes spill their ATP into the Dox liposomes, releasing the Dox from its DNA cage. Second, the walls of the Dox liposomes create an opening in the endosome, spilling their Dox-rich contents into the surrounding cell – leading to cell death.
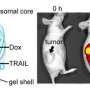
In a mouse model, the researchers found that the new technique significantly decreased the size of breast cancer tumors compared to treatment that used Dox without the nanoscale liposomes.
"This work is somewhat similar to previous research we've done with polymer-based nanogels – but there is a key difference," says Dr. Zhen Gu, senior author of the paper and an assistant professor in the joint biomedical engineering program. "The difference is that this liposome-based technique allows us to introduce additional ATP into the cancer cell, releasing the drug more quickly.
"Being able to adjust ATP levels is important because some cancer cells are ATP deficient," Gu adds. "But this technique would work even in those environments."
More information: The paper, "Enhanced Anticancer Efficacy by ATP-Mediated Liposomal Drug Delivery," is published online in Angewandte Chemie. onlinelibrary.wiley.com/doi/10 … e.201400268/abstract
Abstract
A liposome-based co-delivery system composed of a fusogenic liposome encapsulating ATP-responsive elements with chemotherapeutics and a liposome containing ATP was developed for ATP-mediated drug release triggered by liposomal fusion. The fusogenic liposome had a protein–DNA complex core containing an ATP-responsive DNA scaffold with doxorubicin (DOX) and could release DOX through a conformational change from the duplex to the aptamer/ATP complex in the presence of ATP. A cell-penetrating peptide-modified fusogenic liposomal membrane was coated on the core, which had an acid-triggered fusogenic potential with the ATP-loaded liposomes or endosomes/lysosomes. Directly delivering extrinsic liposomal ATP promoted the drug release from the fusogenic liposome in the acidic intracellular compartments upon a pH-sensitive membrane fusion and anticancer efficacy was enhanced both in vitro and in vivo.
Journal information: Angewandte Chemie
Provided by North Carolina State University