Researchers develop method to process bismuth as catalyst for carbon dioxide reduction
(Phys.org) —A research team led by Joel Rosenthal in the Department of Chemistry and Biochemistry at the University of Delaware has achieved yet another a breakthrough toward the renewable production of solar fuels.
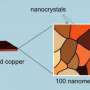
About a year ago, his lab developed an inexpensive bismuth-based catalyst that can utilize electricity generated from solar energy to convert carbon dioxide, a major greenhouse gas, into chemical fuels. Now, they have further developed these catalysts to make them easier and safer to prepare.
"We have also increased their activity such that they can now be used to convert CO2 and renewable energy to conventional fuels with rates and efficiencies that are better than those displayed by traditional precious metal catalysts such as gold and silver," Rosenthal says.
The work is published in a paper in the Journal of the American Chemical Society (JACS), "Efficient Reduction of CO2 to CO with High Current Density Using in Situ or ex Situ Prepared Bi-Based Materials," co-authored by postdoctoral researcher Jonnathan Medina-Ramos and Ph.D. student John DiMeglio.
It was also highlighted in "Science and Technology Concentrates" in Chemical and Engineering News, where expert Clifford Kubiak from the University of California, San Diego referred to the breakthrough as "a very important contribution to the field of electrochemical reduction of CO2."
While renewable energy sources have received a great deal of attention over the past decade, they still account for only a tiny fraction of overall energy production.
"Every second, people across the globe use a combined 17.5 trillion watts of power," Rosenthal says. "That's the equivalent of nearly 200 billion 100-watt light bulbs being lit every second of every day. And that figure has increased approximately 25 percent since the year 2000. The bad news is that this increase has been driven largely by coal, which is the most carbon-intensive energy source."
One of the main barriers to large-scale adoption of renewable energy is supply and demand. Because power generation and use don't always match up well with wind and solar sources, the ability to store energy when it's available and provide energy when it's needed becomes critical. Rosenthal believes CO2-based liquid fuel could be the solution.
"The development of efficient and robust cathode materials that can promote the rapid conversion of CO2 to CO is a key step on the road to the storage and conversion of renewable energy inputs to liquid carbon-based fuels," he says.
When Rosenthal and his team initially discovered that inexpensive bismuth (about $18/kilo) was a viable replacement for silver ($700/kilo) and gold ($40,000/kilo) for CO2 electrocatalysis, they still faced a problem: the process used to prepare the bismuth-based material involved the use of highly acidic solutions, which are corrosive and therefore incompatible with many energy storage platforms.
Now, as reported in the JACS paper, they have discovered that an organic solvent, rather than caustic hydrochloric acid, can be used to form a thin-film catalyst directly on an inexpensive carbon or metal electrode.
More work is needed to work out some challenges in the system, but Rosenthal is confident that bismuth, which is basically a byproduct of processes used to refine other metals, holds great promise in advancing renewable energy technology.
It's a big step up for this metal, whose primary previous use was in the pink stuff that many of us have in our medicine cabinets: Pepto-Bismol.
More information: "Efficient Reduction of CO2 to CO with High Current Density Using in Situ or ex Situ Prepared Bi-Based Materials." Jonnathan Medina-Ramos, John L. DiMeglio, and Joel Rosenthal. Journal of the American Chemical Society Article ASAP. DOI: 10.1021/ja501923g
Journal information: Journal of the American Chemical Society
Provided by University of Delaware