How do our cells move? Liquid droplets could explain
Living cells move; not just bacteria, but also cells in our own bodies. EPFL scientists have discovered a new relationship between the three-dimensional shape of the cell and its ability to migrate. The work has important implications for the fundamental understanding of cell movement and for practical applications like tissue engineering.
'Cell migration' is a broad term for all the processes associated with the movement of cells from one location to another. It lies at the core of biological processes like embryonic development, immune responses and wound healing, but also autoimmune diseases and metastasis of cancerous cells. Cell migration is achieved through the movement of the cell's membrane, which is powered by the action of a protein network inside the cell. However, this interaction is affected by the cell's overall shape, but exactly how this takes place is unclear. Publishing in Current Biology, EPFL scientists have discovered an unexpected link between the 3D shape of the cell and its migration efficiency, and have explained its physics using a simple model of a liquid droplet.
The first step in cell migration occurs when the cell extends its front edge – a process called 'protrusion'. This is driven by the growth of the filaments of the protein actin, which push the cell membrane from inside. Simultaneously, membrane tension controls protrusion by providing resistance protecting the cell from over-extending. But physical laws (e.g. Laplace's law) dictate that the shape of the cell membrane must play a role in the balance between force exerted by actin and the resisting membrane tension. However, this was not taken into account in previous studies, which used simplified 2D models of migrating cells.
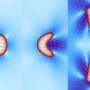
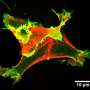
Now, Chiara Gabella and Alexander Verkhovsky at EPFL have determined the relationship between cell protrusion, shape and membrane tension. Using a model cell type taken from fish scales, the researchers developed a fast and simple way to evaluate the 3D shape of migrating cells by observing them in a chamber filled with a fluorescent solution. By applying various treatments to swell, shrink, or stretch the cells, they were able to observe their impact on membrane tension, shape, and protrusion velocity. The treatments only affected the cells' shape and migration speed, but not membrane tension. The overall conclusion was that that the more spherical the cell, the faster it moves.
In order to interpret these unexpected findings, the researchers modeled a migrating cell as a liquid droplet spreading on a surface. "It is well known that a droplet's shape and, in particular, the contact angle that it makes with the surface are determined by the tension forces between the droplet, its environmental medium (e.g. air or a different liquid) and the surface on which it moves", says Gabella. The researchers considered the protruding edge of the cell as such a triple interface, but also added the tension from growing actin filaments inside the cell.
"From this point of view, a more spherical cell means less load for actin filaments to overcome, and therefore faster actin growth and migration", explains Verkhovsky. In support of this idea, the researchers found that the cells were also sensitive to the surface's characteristics just as droplets would be, by slowing down or being pinned at ridges.
"The emphasis of many studies has been on discovering and characterizing individual cellular components", says Verkhovsky. "This is rooted in the common belief that a cell's behavior is determined by intricate networks of genes and proteins". In contrast, this work shows that, despite their enormous molecular complexity, cells can be described as physical objects. The findings point to a new relationship between a cell's shape and its dynamics, and may help to understand how cell migration is guided by the cell's 3D environment.
More information: Gabella C, Bertseva E, Bottier C, Piacentini N, Bornert A, Jeney S, Forró L, Sbalzarini IF, Meister JJ, Verkhovsky AB. Contact Angle at the Leading Edge Controls Cell Protrusion Rate, Current Biology (2014), dx.doi.org/10.1016/j.cub.2014.03.050
Journal information: Current Biology
Provided by Ecole Polytechnique Federale de Lausanne