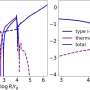
Research group to study interstellar molecules
From April 2014, a new group will study interstellar molecules and use them to explore the entire star and planet formation process at the Max Planck Institute for Extraterrestrial Physics. Newly appointed director Paola Caselli will head the "Centre for Astrochemical Studies at MPE" or CAS@MPE, bringing together theorists, observers and laboratory scientists in one place. This unique combination of expertise is needed to finally make progress on the origin of organic molecules in space as well as our astrochemical origins.
Why are we here? This is probably one of the most intriguing questions – not only in astrophysics. Many scientists (and philosophers) have tried to find answers to this question, and over the past decades there has been some progress in understanding certain aspects of the formation of planets and stars, such as our Sun. It all starts in the interstellar medium, in a relatively dense (in astrophysical terms) cloud of gas and dust. This cloud condenses; a protostar and a protoplanetary disk form; and ultimately a planetary system is born, which might even include a planet in the so-called habitable zone, where liquid water can exist.
"There have been very impressive observations, for example with the IRAM telescopes, the Herschel Space Observatory and ALMA, of dust clouds and star forming regions in our Milky Way, which tell us a lot about the early as well as the late stages of star formation," remarks Paola Caselli, director at the Max Planck Institute for Extraterrestrial Physics (MPE). "Moreover, we have sophisticated theoretical models about the physical processes and dynamics in the clouds, as well as laboratory measurements of molecules, our observing tools as well as building blocks of life. Our aim is to bring everything together in one place: theory, observation and lab experiments, where the experts in each field can learn from each other and everybody has a common aim to work for: to understand how stellar systems form and how chemical complexity evolves during the process of star and planet formation."
Before stellar birth, molecular clouds are very cold, with a temperature of just a few degrees above absolute zero (ca. 10 Kelvin or about -260° Centigrade). This means that one cannot observe them in optical light – they are dark. However, at these low temperatures, molecules can rotate while emitting low energy photons (at radio wavelengths). Thus, astronomers can obtain information about the initial conditions under which stars and planets form via observations at low energies and, at the same time, study the formation and destruction processes of such interstellar (mainly organic) molecules. Theoretical models are being developed to follow the evolution of dust particles and simple molecules under the influence of gravity, magnetic fields and turbulence. The challenge for astronomers lies in finding the right molecule to observe.
"Thanks to astrochemistry, the right molecules can be found and used as unique tools to study the dynamical evolution of interstellar matter: from tenuous clouds to stars, planets and solid bodies such as comets and meteorites. Some of the interstellar molecules are well known, but many still need to be explored as they are not easily produced on Earth," explains Caselli. "This is why we need a lab team as well, who can produce and study still unknown interstellar molecules, thus helping in recognising their spectra during observations and best constrain astrochemical and astrophysical theories." The new "Centre for Astrochemical Studies at MPE" or CAS@MPE will therefore bring together theorists, observers and laboratory scientists in one place to study the molecules and molecular clouds, birth places of stellar systems like our own.
An added complication comes from dust. Interstellar dust grains, even though they are tiny for us (about 1000 times smaller than the average thickness of human hair), are huge compared to the molecules, which themselves range from simple, i.e. made of a few elements (e.g. water (H20), carbon monoxide (CO), ammonia (NH3)) to more complex molecules such as methanol (CH3OH), glycolaldehyde (HCOCH2OH, the simplest sugar), or amino acetonitrile (NH2CH2CN, precursor of glycine, the simplest amino acid). Such pre-biotic molecules have been found in the interiors of comets and meteorites in our Solar system, but the question remains of where they come from.
Molecules can form both in the interstellar gas and then attach to the dust surface (such as CO, the most abundant molecule after molecular hydrogen, H2), or they can form on the dust surface and then evaporate back into the gas phase (such as H2 and H2O). As a result of this interplay between dust and molecules, dust particles in cold regions in space are coated with ice, mostly water ice mixed with simple organic molecules. This ice mantle alters both the behaviour of the dust (for example, dust particles will more easily stick together, thus making the first steps toward planet formation) and that of the gas, as only specific molecules can avoid the frost and are therefore enriched in the gas phase. Meanwhile, large amounts of water ice and organic molecules can be preserved and delivered at later stages, during the formation of planetary systems.
Caselli is confident that "if we bring all this expertise from theory, lab and observation together and focus on certain stages in the whole star formation process, we will be able to bridge the gaps between the various stages and follow the formation of stars, planets and the evolution of chemical complexity through time." Ultimately, if also astrobiologists come on board, this effort might even help to elucidate the human quest on the origin of life.
Provided by Max Planck Society