Flexible battery, no lithium required

(Phys.org) —A Rice University laboratory has flexible, portable and wearable electronics in its sights with the creation of a thin film for energy storage.
Rice chemist James Tour and his colleagues have developed a flexible material with nanoporous nickel-fluoride electrodes layered around a solid electrolyte to deliver battery-like supercapacitor performance that combines the best qualities of a high-energy battery and a high-powered supercapacitor without the lithium found in commercial batteries today.
The new work by the Rice lab of chemist James Tour is detailed in the Journal of the American Chemical Society.
Their electrochemical capacitor is about a hundredth of an inch thick but can be scaled up for devices either by increasing the size or adding layers, said Rice postdoctoral researcher Yang Yang, co-lead author of the paper with graduate student Gedeng Ruan. They expect that standard manufacturing techniques may allow the battery to be even thinner.
In tests, the students found their square-inch device held 76 percent of its capacity over 10,000 charge-discharge cycles and 1,000 bending cycles.
Tour said the team set out to find a material that has the flexible qualities of graphene, carbon nanotubes and conducting polymers while possessing much higher electrical storage capacity typically found in inorganic metal compounds. Inorganic compounds have, until recently, lacked flexibility, he said.
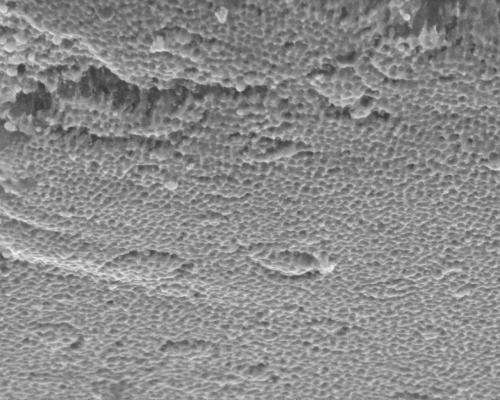
"This is not easy to do, because materials with such high capacity are usually brittle," he said. "And we've had really good, flexible carbon storage systems in the past, but carbon as a material has never hit the theoretical value that can be found in inorganic systems, and nickel fluoride in particular."
"Compared with a lithium-ion device, the structure is quite simple and safe," Yang said. "It behaves like a battery but the structure is that of a supercapacitor. If we use it as a supercapacitor, we can charge quickly at a high current rate and discharge it in a very short time. But for other applications, we find we can set it up to charge more slowly and to discharge slowly like a battery."
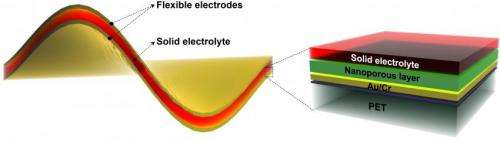
To create the battery/supercapacitor, the team deposited a nickel layer on a backing. They etched it to create 5-nanometer pores within the 900-nanometer-thick nickel fluoride layer, giving it high surface area for storage. Once they removed the backing, they sandwiched the electrodes around an electrolyte of potassium hydroxide in polyvinyl alcohol. Testing found no degradation of the pore structure even after 10,000 charge/recharge cycles. The researchers also found no significant degradation to the electrode-electrolyte interface.

"The numbers are exceedingly high in the power that it can deliver, and it's a very simple method to make high-powered systems," Tour said, adding that the technique shows promise for the manufacture of other 3-D nanoporous materials. "We're already talking with companies interested in commercializing this."
Rice graduate student Changsheng Xiang and postdoctoral researcher Gunuk Wang are co-authors of the paper.
The Peter M. and Ruth L. Nicholas Postdoctoral Fellowship of the Smalley Institute for Nanoscale Science and Technology and the Air Force Office of Scientific Research's Multidisciplinary University Research Initiative supported the research.
More information: "Flexible Three-Dimensional Nanoporous Metal-Based Energy Devices." Yang Yang, et al. J. Am. Chem. Soc., Article ASAP. DOI: 10.1021/ja501247f
Journal information: Journal of the American Chemical Society
Provided by Rice University