Standardizing net quantity statements for aerosol contents

The work of NIST can be found in many unexpected places in American life—including store shelves containing different kinds of aerosol products. So perhaps it's not surprising that PML's Office of Weights and Measures (OWM) was recently called upon to help resolve a dispute over the way such products are labeled and sold.
It all began a few years ago in Massachusetts when a state official was out in the field and noticed a spray can on which the net quantity statement was apparently mislabeled. Under the Uniform Packaging and Labeling Regulation found in NIST Handbook 130 (Uniform Laws and Regulations in the Areas of Legal Metrology and Engine Fuel Quality), which most states adopt or use as the basis for their regulation, aerosols and similar pressurized containers must be sold by net weight. This one was sold by fluid volume. State officials notified the manufacturer that the net-weight requirement applied to all aerosols and similar pressurized containers.
But the company's products used "bag-on-valve" (BOV) technology. "And a lot of BOV manufacturers do not regard themselves as 'similar'," says David Sefcik of OWM, "because their technology is different."
Many familiar aerosol products contain a mixture of the active ingredient and the propellant gas. Both are expelled at the same time. But in BOV containers – which are used for a range of products including sunblock sprays, deodorants, window cleaners, insecticides, furniture polish, and automotive lubricants – the active ingredient is confined in a sealed bag within the can. The bag is surrounded by pressurized gas, which never leaves the container.
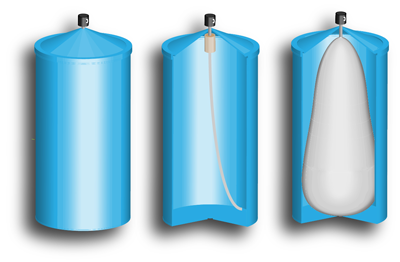
"The manufacturer argued that because its products don't expel the propellant, it should continue to use its existing net-volume declaration," Sefcik says. "The state contacted us. We recommended that net weight be used for BOV products, and encouraged them to bring the issue before the National Conference on Weights and Measures (NCWM), which makes the ultimate decisions about such questions."
A couple of years passed, and industry representatives, two major trade associations, and other interested parties were not able to reach a consensus on the matter. As a result, a significant problem remained: Products using different dispensing technology that cannot be easily distinguished at the point of sale had the net quantity labeled in two different ways, making consumer value comparisons difficult, if not impossible.
"Meanwhile," Sefcik says, "we had begun to examine the subject more closely. The issue crossed over several different federal agencies, and we soon realized that the agencies' regulations and guidance were not entirely consistent among themselves."
For example, the Uniform Labeling and Packaging Regulation, adopted or used as a basis of adoption by 45 states as of this year, clearly calls for the declaration of quantity in an "aerosol package and on a similar pressurized containers" to be expressed in terms of weight. The Food and Drug Administration (FDA), however, allows a "net quantity" of "weight or volume." The Environmental Protection Agency (EPA) guidance stipulates that "the net contents of BOV pesticide products must be expressed in terms of both liquid measure and weight." And the Federal Trade Commission (FTC) requires that the package state the "net quantity" of the contents, but does not specify whether it should be by weight or volume.
"So the Laws and Regulations committee of the NCWM recommended that NIST facilitate a meeting of all the concerned parties" Sefcik says. "And that's what we did – invite all stakeholders together, in an effort to educate them regarding the issue and to reach a consensus on the proper method of sale." *
On January 9 of this year, OWM's Sefcik and colleagues hosted a meeting of representatives from various aerosol industry groups, trade associations, and federal agencies (FDA, FTC, EPA, and the Consumer Product Safety Commission). After multiple presentations and a thorough discussion, "we were able to reach a unanimous consensus that the products should be sold by weight," Sefcik says.
The report on that meeting became the basis for a recommendation to the NCWM that products using BOV technology be sold by weight in accordance with current federal and state regulations. It was also recommended that current language in NIST Handbook 130's section on aerosols and pressurized containers be revised and clarified. Proposed language will be voted on in July at the NCWM's 99th annual meeting in Detroit. It is expected that manufacturers will be given a grace period of about three years to re-label their products, and that federal agencies will act to make their regulations and guidance more consistent.
"It's as fair as it can be," Sefcik says. "The BOV companies won't have to distinguish themselves from aerosols. And consumers will be able to make rational purchasing decisions and value comparisons based on a common method of sale."
Provided by National Institute of Standards and Technology




















