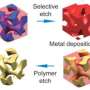
Nanoparticle networks' design enhanced by theory
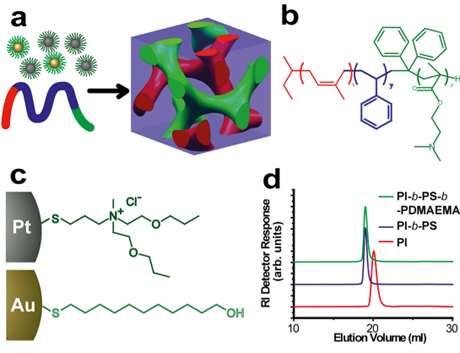
For close to two decades, Cornell scientists have developed processes for using polymers to self-assemble inorganic nanoparticles into porous structures that could revolutionize electronics, energy and more.
This process has now been driven to an unprecedented level of precision using metal nanoparticles, and is supported by rigorous analysis of the theoretical details behind why and how these particles assemble with polymers. Such a deep understanding of the complex interplay between the chemistry and physics that drive complex self-assembly paves the way for these new materials to enter many applications, from electrocatalysis in fuel cells to voltage conductance in circuits.
Ulrich Wiesner, the Spencer T. Olin Professor of Materials Science and Engineering, led what is probably the most comprehensive study to date of block copolymer nanoparticle self-assembly processes. The study was published online Feb. 21 in Nature Communications.
From the outside, the process looks simple enough. Begin with platinum and gold particles that grow from a precursor. A chemical called a ligand coats the particles and precisely controls their size. Add to this designed molecules called block copolymers – long chains of two or three organic materials. The polymers combine with the platinum and gold nanoparticles, all of which assemble into ordered, cubic, three-dimensional structures. Etch away the polymer, and what's left are scores of nanoparticles forming porous 3-D cubic networks.
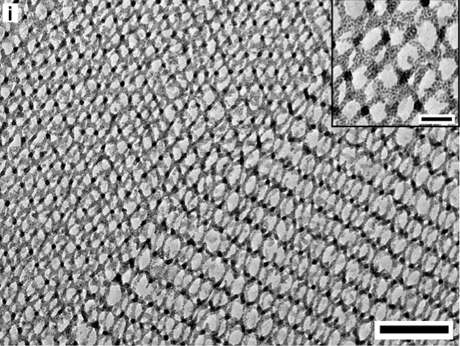
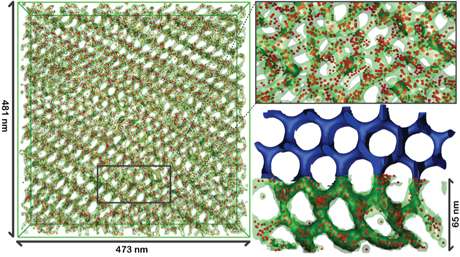
Each step – from the exact structure of the ligands, to the synthesis of the polymers – requires precise chemistry and detailed understanding of each material's role. The Nature Communications analysis drew on the expertise of collaborators in electron tomography, energy dispersive microscopy and percolation theory. For example, collaborators from the Japan Science and Technology Agency used electron tomography to map the location of every single particle in the samples, which then could be compared with theoretical predictions. The result is a comprehensive set of design criteria that could lead to readying these particle networks for larger scale solution processing.
"Not only can we make these materials, but through electron tomography in particular, we can analyze these structures at a depth that just has not been done before," Wiesner said. "The comparison with theory allows us to fully understand the physical mechanisms by which these structures are formed."
Why pay such attention to these self-assembled nanoparticle networks? They're made in a way that would never happen in nature or by conventional laboratory means. They are uniformly porous with high surface area and, therefore, are highly catalytic and potentially useful for energy applications.
Perhaps best of all, working with polymers means cost-effective, large-scale processing could be a snap.
Several decades of polymer science has given the world efficient scalability unsurpassed in the materials world – think plastics production. Wiesner and colleagues have proven the concept of self-assembled metal nanoparticles using block copolymer-based solution processing that goes beyond the "glass vial in a lab," Wiesner said.
"Now that we understand how it all works, our process lends itself easily to larger-scale production of such materials," he said.
More information: "Linking experiment and theory for three-dimensional networked binary metal nanoparticle–triblock terpolymer superstructures." Zihui Li, et al. Nature Communications 5, Article number: 3247 DOI: 10.1038/ncomms4247
Journal information: Science , Nature Communications
Provided by Cornell University