Local factors cause dramatic spikes in coastal ocean acidity

(Phys.org) —A new Duke University-led study has documented dramatic, natural short-term increases in acidity in a North Carolina estuary.
"The natural short-term variability in acidity we observed over the course of one year exceeds 100-year global predictions for the ocean as a whole and may already be exerting added pressure on some of the estuary's organisms, particularly shelled organisms that are especially susceptible to changes in pH," said Zackary I. Johnson, Arthur P. Kaupe Assistant Professor of Molecular Biology at Duke's Nicholas School of the Environment
The short-term spikes in estuarine acidity were driven by changes in temperature, water flow, biological activity and other natural factors, and are occurring in addition to the long-term acidification taking place in Earth's oceans as a result of human-caused climate change.
"For vulnerable coastal marine ecosystems, this may be adding insult to injury," said Johnson, who was lead author of the study.
When the effects of long-term ocean acidification and short-term natural variation combine, they can create "extreme events" which may be especially harmful to coastal marine life, he said.
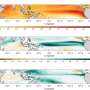
The study was conducted at the Pivers Island Coastal Observatory at the Duke Marine Lab in Beaufort, N.C., as part of a long-term coastal monitoring program. Researchers collected seawater samples from Beaufort Inlet weekly for a year and on a daily and hourly basis for shorter periods to track changes in the water's pH and dissolved inorganic carbon on multiple time scales.
Numerous studies have shown that increasing amounts of atmospheric carbon dioxide from human sources are finding their way into the world's oceans. When the carbon dioxide dissolves in seawater, it reduces the water's pH and the ability of organisms to form calcium carbonate minerals that are the building blocks of many species' shells and skeletons. This process is known as ocean acidification.
If current trends continue, experts predict that the mean ocean pH will decrease by about 0.2 units over the next 50 years. A drop of that magnitude could have far-reaching impacts on ocean ecosystems and organisms.
"We may see significant changes in biological processes such as primary production. Some organisms, such as phytoplankton, may benefit. Many others, including shelled organisms and corals, will not," said Dana Hunt, assistant professor of microbial ecology, who co-authored the new study.
The Duke team's analysis showed that a wide range of natural variables, including changes in temperature, algal production and respiration, and water movement caused by tides and storms, triggered sharp spikes in the inlet's acidity. Some changes occurred over the course of a season; others took place on a daily or hourly basis.
"Understanding to what extent pH naturally varies in coastal ecosystems worldwide will be essential for predicting where and when the effects of increasing ocean acidity will be most profound, and what organisms and ecosystems may be most affected," Hunt said. "Our research demonstrates we have to take into account a wide range of environmental variables, not just pH."
The study was published online this week in the peer-reviewed open-access journal PLOS ONE.
More information: "Dramatic Variability of the Carbonate System at a Temperate Coastal Ocean Site (Beaufort, North Carolina) is Regulated by Physical and Biogeochemical Processes on Multiple Timescales," by Zackary I. Johnson, Benjamin J. Wheeler, Sara K. Blinebry, Christina M. Carlson, Christopher S. Ward, Dana E. Hunt. Published Dec. 17, 2013, in PLOS ONE. DOI: 10.1371/journal.pone.0085117
Journal information: PLoS ONE
Provided by Duke University