New small-molecule catalyst does the work of many enzymes
Researchers report that they have created a man-made catalyst that is an "enzyme mimic." Unlike most enzymes, which act on a single target, the new catalyst can alter the chemical profiles of numerous types of small molecules. The catalyst – and others like it – will greatly speed the process of drug discovery, the researchers say.
Their findings appear in the Journal of the American Chemistry Society.
Most enzymes are large proteins that act on only one molecular target, said University of Illinois chemistry professor M. Christina White, who conducted the study with graduate student Paul Gormisky. Enzymes generally modify the chemical profiles of their targets to dismantle them or to enable them to perform specific functions.
One key modification involves replacing a carbon-hydrogen (C-H) bond with a carbon-oxygen (C-O-H or C=O) bond. These reactions, called oxidations, are essential to countless processes in the body, from drug detoxification to biosynthesis.
The new catalyst can oxidize specific C-H bonds on many different targets. This will greatly streamline the process of modifying known molecules in new ways, a key part of drug discovery, White said.
"The main cost of drugs isn't making the drug, it's actually discovering the drug, in part because there aren't good ways to diversify molecules," she said. "So if you have one molecule of interest that you'd like to modify, you often have to resynthesize the whole thing. It's not efficient."
The other option is to develop a new enzyme for every modification you want to make, she said.
"Let's say someone in industry has some kind of medicinal compound and they want to oxidize it in a way that will give it a different or improved biological function," she said. "Currently, this may be accomplished either by using an enzyme that had been specifically engineered for that molecule, or, more commonly, through a long synthetic process that could take months to complete."
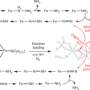
The new catalyst (called iron CF3-PDP) can accomplish one of these alterations in about half an hour, she said.
This catalyst and a previous one from White's lab (called iron PDP) have been designed to oxidize specific types of C-H bonds. Iron PDP goes after the most electron-rich C-H bond on a molecule, while the new catalyst targets the most electron-rich C-H bond that also is the least encumbered by nearby atoms.
The specificity of the new catalysts allows the researchers to use computational methods and modeling to predict which bonds the catalysts will alter, Gormisky said.
"The other breakthrough here is that this model could be very generally applicable not just to our catalysts, but this whole genre of catalysts that do C-H oxidations," White said.
The new catalyst has some limitations. It only oxidizes certain bonds on linear or cyclic molecules, and it doesn't work on aromatic rings.
"But with the two new catalysts you can quickly and efficiently oxidize up to two different sites on one molecule," she said. She and her colleagues hope to create "a whole toolbox of these things" to oxidize potentially any C-H bond on any molecule, she said.
More information: "Catalyst Controlled Aliphatic C-H Oxidations With a Predictive Model for Site Selectivity," pubs.acs.org/doi/abs/10.1021/ja407388y
Provided by University of Illinois at Urbana-Champaign