Pass the salt: Common condiment could enable new high-tech industry
Chemists at Oregon State University have identified a compound that could significantly reduce the cost and potentially enable the mass commercial production of silicon nanostructures – materials that have huge potential in everything from electronics to biomedicine and energy storage.
This extraordinary compound is called table salt.
Simple sodium chloride, most frequently found in a salt shaker, has the ability to solve a key problem in the production of silicon nanostructures, researchers just announced in Scientific Reports, a professional journal.
By melting and absorbing heat at a critical moment during a "magnesiothermic reaction," the salt prevents the collapse of the valuable nanostructures that researchers are trying to create. The molten salt can then be washed away by dissolving it in water, and it can be recycled and used again.
The concept, surprising in its simplicity, should open the door to wider use of these remarkable materials that have stimulated scientific research all over the world.
"This could be what it takes to open up an important new industry," said David Xiulei Ji, an assistant professor of chemistry in the OSU College of Science. "There are methods now to create silicon nanostructures, but they are very costly and can only produce tiny amounts.
"The use of salt as a heat scavenger in this process should allow the production of high-quality silicon nanostructures in large quantities at low cost," he said. "If we can get the cost low enough many new applications may emerge."

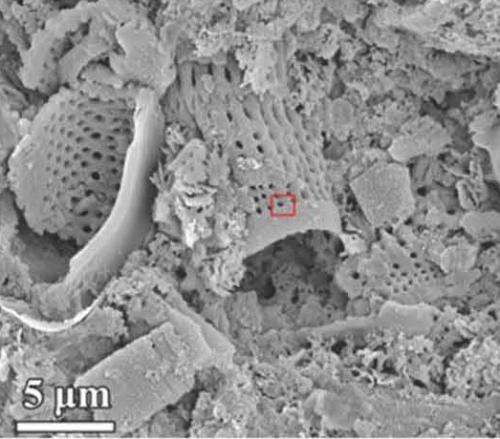
Silicon, the second most abundant element in the Earth's crust, has already created a revolution in electronics. But silicon nanostructures, which are complex structures much smaller than a speck of dust, have potential that goes far beyond the element itself.
Uses are envisioned in photonics, biological imaging, sensors, drug delivery, thermoelectric materials that can convert heat into electricity, and energy storage.
Batteries are one of the most obvious and possibly first applications that may emerge from this field, Ji said. It should be possible with silicon nanostructures to create batteries – for anything from a cell phone to an electric car – that last nearly twice as long before they need recharging.
Existing technologies to make silicon nanostructures are costly, and simpler technologies in the past would not work because they required such high temperatures. Ji developed a methodology that mixed sodium chloride and magnesium with diatomaceous earth, a cheap and abundant form of silicon.
When the temperature reached 801 degrees centigrade, the salt melted and absorbed heat in the process. This basic chemical concept – a solid melting into a liquid absorbs heat – kept the nanostructure from collapsing.
The sodium chloride did not contaminate or otherwise affect the reaction, researchers said. Scaling reactions such as this up to larger commercial levels should be feasible, they said.
The study also created, for the first time with this process, nanoporous composite materials of silicon and germanium. These could have wide applications in semiconductors, thermoelectric materials and electrochemical energy devices.
More information: www.nature.com/srep/2013/13071 … /full/srep02222.html
Journal information: Scientific Reports
Provided by Oregon State University