Study suggests second life for possible spintronic materials
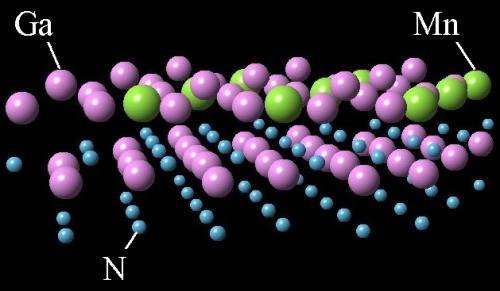
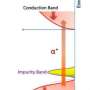
Ten years ago, scientists were convinced that a combination of manganese and gallium nitride could be a key material to create spintronics, the next generation of electronic devices that operate on properties found at the nanoscale. But researchers grew discouraged when experiments indicated that the two materials were as harmonious as oil and water.
A new study led by Ohio University physicists suggests that scientists should take another look at this materials duo, which once was heralded for its potential to be the building block for devices that can function at or above room temperature.
"We've found a way—at least on the surface of the material—of incorporating a uniform layer," said Arthur Smith, a professor of physics and astronomy at Ohio University who leads the international collaboration of Argentinian and Spanish researchers.
The scientists made two important changes to create the material merger, they report in the journal Physical Review B. First, they used the nitrogen polarity of gallium nitride, whereas conventional experiments used the gallium polarity to attach to the manganese, Smith explained. Second, they heated the sample.
At lower temperatures (less than 105 degrees Celsius), the manganese atoms "float" on the outer layer of gallium atoms. When the scientists raised the temperature about 100 degrees Celsius, Smith said, the atoms connected to the nitrogen layer underneath, creating a manganese-nitrogen bond. This bond remains stable, even at very high temperatures.
The theoretical scientists accurately predicted that a "triplet" structure of three manganese atoms would form a metastable structure at low temperatures, Smith said. But at higher temperatures, those manganese atoms break apart and bond with nitrogen. Valeria Ferrari of the Centro Atómico Constituyentes said her group performed quantum mechanical simulations to test which model structures have the lowest energy, which suggested both the trimer structure and the manganese-nitrogen bonded structure.
Now that scientists have shown that they can create a stable structure with these materials, they will investigate whether it has the magnetic properties at room temperature necessary to function as a spintronic material.
Journal information: Physical Review B
Provided by Ohio University