January 2, 2013 feature
Sodium-air battery offers rechargeable advantages compared to Li-air batteries
(Phys.org)—Over the past few years, Li-air batteries (more precisely, Li-oxygen batteries) have become attractive due to their theoretical ability to store nearly as much energy per volume as gasoline. The key to this high energy density is the "air" part, since the batteries capture atmospheric oxygen to use in the cathode reaction instead of storing their own oxidizing agent. However, Li-air batteries have conventionally been single-use cells since they cannot be recharged, which significantly limits their applications. Now in a new study, scientists have found that replacing the lithium anode with a sodium anode may offer an unexpected path toward making metal-air batteries rechargeable while still offering a relatively high energy density.
The researchers, led by Professor Jürgen Janek and Dr. Philipp Adelhelm at the Institute of Physical Chemistry at Justus-Liebig-University Gießen in Gießen, Germany, have published their paper on rechargeable sodium-air batteries in a recent issue of Nature Materials.
Li-air batteries' greatest appeal is their high theoretical energy density (about 3,458 Wh kg-1), which is several times higher than that of Li-ion batteries, the most commonly used battery in electric vehicles today. However, whereas Li-ion batteries can be recharged many times while retaining most of their capacity, most Li-air batteries cannot be recharged at all. In 2011, several international groups discovered that this irreversibility is due to the instability of the Li-air battery's electrolyte and other cell components in the presence of the reactive superoxide radical O2-, which forms as a first step during cell discharge. Only recently, improvements in rechargeability have been achieved by using gold electrodes, but the system still suffers from poor energy efficiency and large overpotentials in which some energy is lost as heat.
In the new study, the researchers demonstrated that a sodium-air (Na-air) cell does not suffer from the same negative effects on the electrolyte and energy efficiency as a Li-air cell does. This is because, while lithium and sodium are closely related chemically, they each react very differently with oxygen. When lithium reacts with oxygen, it forms LiO2 (lithium oxide), which is highly unstable and found only as an intermediate species in Li-air batteries, after which it turns into Li2O2. On the other hand, sodium and oxygen form NaO2 (sodium superoxide), a more stable compound. Since NaO2 doesn't decompose, the reaction can be reversed during charging.
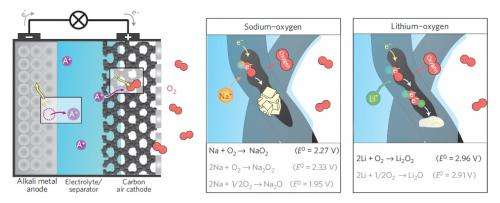
The scientists demonstrated the reversibility of Na-air cells in their experiments. Using several techniques, including Raman spectroscopy and X-ray diffraction, the scientists confirmed that NaO2 is indeed produced during discharge, that Na and O2 are separated during charging, and that the cycle can be repeated.
"We could demonstrate that by replacing lithium with sodium, the cell reaction proceeds in an unexpected and beneficial manner," Adelhelm told Phys.org. "The cell discharge and charge process is kinetically favored, which means that the formation and decomposition of NaO2 is very energy-efficient."
Although this evidence of reversibility is a promising step, the reaction is far from ideal. While Na-air batteries have a theoretical energy density of 1,605 Wh kg-1, which is significantly higher than that of Li-ion batteries, it is still only about half that of Li-air batteries. And even though the Na-air batteries can be charged and discharged several times, the capacity decreases after each cycle, with negligible energy storage after eight cycles. The researchers are currently investigating the processes that limit battery lifetime.
Still, Na-air batteries have some attractive characteristics. One advantage of the Na-air battery demonstrated here is its very low overpotential, which is three or four times lower than for any Li-air or Na-air battery previously reported, resulting in fewer losses. In addition, sodium is the sixth most abundant element on Earth, while lithium resources are much more limited.
"Our results are also important from another perspective," Adelhelm said. "NaO2 is chemically very difficult to synthesize. High temperatures, pressures and long reaction times are needed. In our battery, NaO2 forms instantly at room temperature and ambient pressure. Possibly, also other chemical compounds could be prepared this way."
Overall, Adelhelm hopes that Na-air batteries may serve as one more option to turn to for future energy storage applications.
"A broad variety of electrochemical energy storage devices with different properties is needed for future mobile and stationary applications," he said. "In short, sodium's abundance could be an important cost advantage over lithium; however, for otherwise identical batteries, the 'lithium version' will always provide the higher energy density. But any working metal/air battery will provide a higher energy density than current Li-ion batteries."
More information: Pascal Hartmann, et al. "A rechargeable room-temperature sodium superoxide (NaO2) battery." Nature Materials. DOI: 10.1038/NMAT3486
Journal information: Nature Materials
Copyright 2013 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of Phys.org.


















