Researchers identify critical operating temperature of catalytic converters

(Phys.org)—Catalytic converters work poorly if they have not yet warmed up. Tiny metal particles in a catalytic converter require a minimum temperature to function efficiently. At the Vienna University of Technology, thanks to a new measuring method, it has now become possible to examine many different types of these particles at the same time. Reliable information regarding what it is exactly that the efficiency of catalytic converters depends on has thus been obtained for the first time.
Low ignition temperature desired
"A large part of the pollutant emissions from an engine are generated immediately after start-up, while the catalytic converter is still cold", explains Prof. Günther Rupprechter from the Institute of Materials Chemistry at Vienna University of Technology. "Only when a certain temperature is exceeded does what is known as catalytic ignition occur and the catalytic converter functions with high efficiency." Complex and expensive catalytic converter heating systems have already been developed in order to reach this critical temperature as quickly as possible. It would, however, save both money and energy to build a catalytic converter that already functions well at the lowest possible temperatures.
Straight or slanting? It all depends on the angle
The critical temperature that the catalytic converter must reach depends on the material used: the precious metals platinum and palladium are used particularly often in catalytic converters. However, the crystallographic orientation of the surfaces of the tiny metal granules also plays an important role. Crystals can be cut in different highly-specific directions – this process is familiar from cut precious stones. Even the surfaces of naturally grown crystals are formed in differing directions and the orientation of these surfaces determines the chemical behaviour of the crystals. "It is apparent that surfaces with differing crystallographic directions require different temperatures for catalytic ignition", explains Prof. Yuri Suchorski, who works with Prof. Rupprechter.
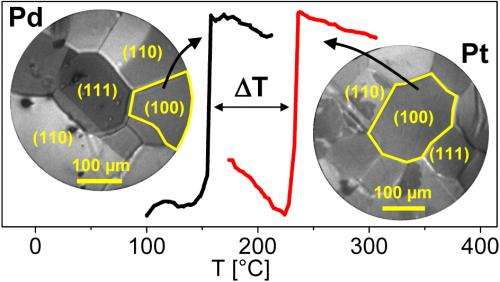
Many measurements in one experiment
Previously, it had hardly been possible to investigate this effect in detail: a catalytic converter is made up of countless tiny granules. "Until now, it has only been possible to measure the superimposed activity of all these differently-oriented granules", says Rupprechter. However, Rupprechter and his team have now been able to use a photoemission electron microscope based on Einstein's famous "photo effect" to analyse the ignition temperatures of the individual metal granules whilst the reaction is occurring. For this, a film was used, on which many tiny crystals – with diameters of only around 100 micrometres – were arranged closely together. The directions of the crystals were randomly distributed, thereby allowing different crystal variants to be investigated in a single experiment.
Under the microscope, the temperature of the film was slowly increased, actually demonstrating that the catalytic ignition took place at different temperatures depending on the direction of orientation. "It is important to us to be able to investigate different crystal grains in close proximity and under identical conditions during a single experiment," explain the researchers. "If you carry out several experiments one after another, it is never possible to perfectly reproduce the external conditions so that the individual measurements are directly comparable."
With the new findings, it is now possible to perform targeted searches for manufacturing processes for catalytic converters with lower ignition temperatures. "We now know that palladium works better than platinum, and we know which crystallographic direction promises the lowest ignition temperature", says Günther Rupprechter. Now it should also be possible to implement these findings in technology, in order to build catalytic converters which take effect in cars as soon as possible after start-up.
More information: D. Vogel, Ch. Spiel, Y. Suchorski, A. Trinchero, R. Schlögl, Henrik Grönbeck, G. Rupprechter, "Local light-off in catalytic CO oxidation on low-index Pt and Pd surfaces: a combined PEEM, MS and DFT study", Angewandte Chemie International Edition, 51 (2012) 10041–10044.
Journal information: Angewandte Chemie International Edition
Provided by Vienna University of Technology
















