Graphene films—highly resistant to damage—could protect metals in harsh environments
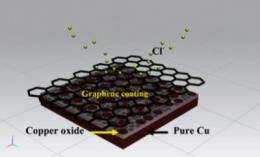
(Phys.org)—A coating so thin it's invisible to the human eye has been shown to make copper nearly 100 times more resistant to corrosion, creating tremendous potential for metal protection even in harsh environments.
In a paper published in the September issue of Carbon, researchers from Monash University and Rice University in the USA say their findings could mean paradigm changes in the development of anti-corrosion coatings using extremely thin graphene films.
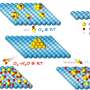
Graphene is a microscopically thin layer of carbon atoms. It is already in use in such things as smartphone screens, and is attracting research attention for its possibilities as a means of increasing metal's resistance to corrosion.
"We have obtained one of the best improvements that have been reported so far," said study co-author Dr Mainak Majumder. "At this point we are almost 100 times better than untreated copper. Other people are maybe five or six times better, so it's a pretty big jump."
Dr Parama Banerjee, who performed most of the experiments for this study, said graphene had excellent mechanical properties and great strength.
The polymer coatings that are often used on metals can be scratched, compromising their protective ability, but the invisible layer of graphene – although it changes neither the feel nor the appearance of the metal – is much harder to damage.
"I call it a magic material," Dr Banerjee said.
The researchers applied the graphene to copper at temperatures between 800 and 900 degrees, using a technique known as chemical vapour deposition, and tested it in saline water.
"In nations like Australia, where we are surrounded by ocean, it is particularly significant that such an atomically thin coating can provide protection in that environment," Dr Banerjee said.
Initial experiments were confined to copper, but Dr Banerjee said research was already under way on using the same technique with other metals.
This would open up uses for a huge range of applications, from ocean-going vessels to electronics: anywhere that metal is used and at risk of corrosion. Such a dramatic extension of metal's useful life could mean tremendous cost savings for many industries.
The process is still in the laboratory-testing stage, but Dr Majumder said the group was not only looking at different metals, but also investigating ways of applying the coating at lower temperatures, which would simplify production and enhance market potential.
Journal information: Carbon
Provided by Monash University