Unusual reaction eschews high temperatures and water to lock carbon dioxide away
(Phys.org) -- When it comes to reducing the impact of the energy we use to cool our homes and power our computers, one option is to remove gaseous carbon dioxide (CO2), pump it into underground reservoirs, and have it become part of the mineral formations. If the CO2 doesn't react, it remains in a state that could be released by drilling or earthquakes, defeating the purpose of sequestering the carbon away from the atmosphere. Keeping the CO2 trapped by transforming into minerals, called carbonation reactions, take place much more readily at high temperatures. But, scientists at Pacific Northwest National Laboratory discovered a reaction that breaks the rules. At relatively low temperatures and while recycling the water it needs, this reaction transforms CO2 into the mineral magnesite.
Our nation needs electricity for industrial and personal use; the electricity that comes from power plants that burn coal and other fossil fuels pumps CO2 into the atmosphere. These emissions contribute to changes in weather patterns, affecting crops, water supplies, and air quality. Capturing and storing CO2 could mitigate its effects. By understanding the fundamental sequestration reactions, scientists can inform policymakers and others about different options. Industries that form minerals could also benefit from the team's discovery, as it offers a pathway to make valuable materials with lower energy costs.
This study drew upon the team's desire to recycle water during carbonation to increase the amount of mineral carbonation. Water drives the reactions, but it can also be consumed in the process.
"If the water was re-liberated, you could achieve significant mineral formation with just a small amount of water," said Dr. Kevin Rosso, a geochemist and PNNL Fellow who worked on this study.
To recycle the water, the scientists took on a fairly difficult challenge: create minerals that are not bound to water molecules in an environment where the minerals need water to form. Specifically, they wanted water-free or anhydrous magnesite. The water-packed or hydrous form is very water soluble and hence is not effective at sequestering CO2 in mineral form. The anhydrous form is much better for carbon storage. Conventional views state that creating the anhydrous form requires high temperatures, which often do not occur in carbon storage reservoirs.
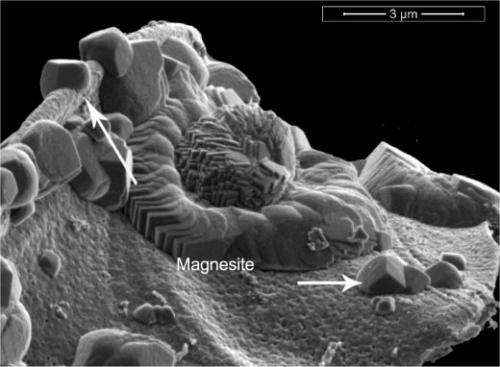
The team began with highly reactive nanometer-sized particles of forsterite, MgSiO4. Forsterite-rich minerals are the most abundant minerals in the Earth's mantle for the first 250 miles or so. Using a host of instruments at EMSL, and unique capabilities developed within other parts of PNNL, the team studied the reaction as it happened and after it was done. They ran experiments at 95°F and 122°F.
They introduced water-saturated supercritical CO2 to the particles. This form of CO2 behaves like a liquid and a gas; it is easier to pump than the gaseous form. They examined the forsterite surface using scanning electron microscopy and characterized the molecules formed using confocal Raman spectroscopy, nuclear magnetic resonance spectroscopy, and energy-dispersive x-ray spectroscopy. In addition, the team confirmed their results using a reaction cell approach and the in situ x-ray diffraction spectrometer at PNNL.
"We had to present overwhelming evidence that this reaction had occurred at these low temperatures," said Felmy. "Nobody has been able to form this phase from aqueous solutions at these low temperatures, but we have."
The results show that within 3 to 4 days, the forsterite, water, and CO2 react to form a mixture of two magnesium-based minerals: nesquehonite, which contains water, and anhydrous magnesite, which does not. Water is continually used and released in the process, with the water driving the reaction. Over 14 days, magnesite and a highly porous amorphous silica phase are produced. After two weeks of reacting, the forsterite is transformed into the magnesite.
The team at PNNL is defining the molecular mechanism that forms the magnesite. The team is determining the precursors to the reaction, specifically the nucleation clusters.
More information: AR Felmy, O Qafoku, BW Arey, JZ Hu, MY Hu, HT Schaef, ES Ilton, NJ Hess, CI Pearce, J Feng, and KM Rosso. 2012. "Reaction of Water-Saturated Supercritical CO2 with Forsterite: Evidence for Magnesite Formation at Low Temperatures." Geochimica et Cosmochimica Acta 91:271-282. DOI:10.1016/j.gca.2012.05.026
Journal information: Geochimica et Cosmochimica Acta
Provided by Pacific Northwest National Laboratory