New structural information on functionalization of gold nanoparticles
Nanometre-scale gold particles are currently intensively investigated for possible applications as catalysts, sensors, biolabels, drug delivery devices, biological contrast agents and as components in photonics and molecular electronics. The particles are prepared in a solution from gold salts and their reactive gold cores can be stabilized with various organic ligands. Particularly stable particles can be synthesized by using organothiol ligands that have a strong chemical interaction to gold, producing precise compositions in the size range of 1 to 3 nanometres. Modification of the protecting molecular overlayer is a key step in almost all applications. A detailed structural atomistic understanding of the processes of the exchange reaction has been lacking.
Now, professors Chris Ackerson in the Colorado State University in Ft. Collins, USA, and Hannu Häkkinen at the Nanoscience Center of the University of Jyväskylä, Finland, report the first structural study on the atomistic processes of a ligand-exchange reaction of a well-defined gold nanoparticle that has 102 gold atoms and 44 ligand sites in the molecular overlayer. The study was published in the Journal of the American Chemical Society on 21 July 2012 [1]. Prof. Häkkinen's work is funded by the Academy of Finland and prof. Ackerson's work is funded by the Colorado State University and the American Federation for Aging Research.
The studied particle has a chemical formula of Au102(p-MBA)44 and it was made by using a water-soluble thiol (para – mercapto benzoic acid, p-MBA) as the stabilizing molecule. The X-ray crystal structure of this particle was first reported as the cover article of Science in 2007 by the group of Roger D. Kornberg from Stanford University [2]. Häkkinen led an international team of researchers that published a theoretical analysis of this and other thiol-stabilized gold nanoparticles in 2008 in the Proceedings of the National Academy of Sciences [3].
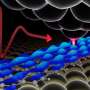
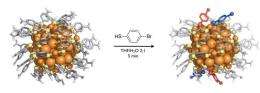
In the new study, Ackerson's group succeeded in making heterogeneous crystals of samples of Au102 particles that had undergone a ligand-exchange reaction where the p-MBA thiols in the molecular overlayer had been partially exchanged to a similar thiol containing a Bromine atom, the so-called para – bromo benzene thiol (p-BBT), under a fast 5-minute reaction. The analysis of the heterogeneous crystals showed which ligand sites in the overlayer are the most likely to be changed during the short reaction time, i.e., from which sites the exchange process starts. Surprisingly, only 4 sites out of the 44 possibilities showed occupation by the exchanged ligand (see Figure). Theoretical analysis performed by Häkkinen's group gave insight into the atomistic details of possible reaction mechanisms. Evidence from experiment and theory indicates that the Au102(p-MBA)44 nanoparticle has a thiol overlayer where almost every thiol ligand site has its own reaction rate due to a highly heterogeneous structure of the overlayer. "The Au102(p-MBA)44 nanoparticle has a structure reminiscent of a protein, with a rigid inorganic gold core analogous to the alpha-carbon backbone of a protein core and chemically modifiable functional groups in the low-symmetry molecular overlayer", says prof. Ackerson. "When ligand exchange reactions are better understood, we hope to fully control the surface functionalization of the Au102 and similar water-soluble gold nanoparticles. The implications in biology for a fully controllable synthetic surface the size of a protein are profound", says prof. Häkkinen.
More information: 1. C.L. Heinecke, T.W. Ni, S.A. Malola, V.P. Mäkinen, O.A. Wong, H. Häkkinen and C.J. Ackerson, "Structural and theoretical basis for ligand exchange on thiolate monolayer protected gold nanoclusters", J. Am. Chem. Soc., published online July 21, 2012 (pubs.acs.org/doi/abs/10.1021/ja3032339).
2. P.D. Jadzinsky, G. Calero, C.J. Ackerson, D.A. Bushnell and R.D. Kornberg, "Structure of a thiol monolayer-protected gold nanoparticle at 1.1. Ångstrom resolution", Science 318, 430 (2007) (www.sciencemag.org/content/318/5849/430.abstract).
3. M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck, H. Häkkinen, "A unified view of ligand-protected gold clusters as superatom complexes", Proc. Natl. Acad. Sci. (USA) 105, 9157 (2008) (www.pnas.org/content/105/27/9157).
Journal information: Journal of the American Chemical Society , Proceedings of the National Academy of Sciences
Provided by Academy of Finland