Borrowing from nature to produce highly structured biomimetic materials
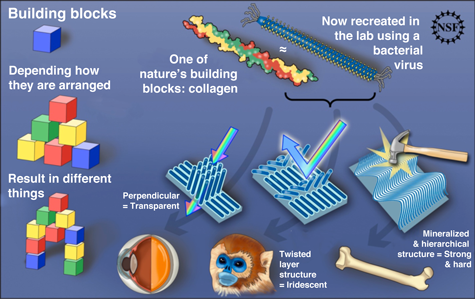
Natural biological tissues are often hierarchically structured, and these structures appear to correlate strongly with tissue properties and functionalities. Finding out how a tissue self-assembles from cellular proteins has captured the interest of many biologists and material scientists who are interested in borrowing from nature’s bag of tricks to synthesize artificial materials with desired properties. A team of Berkeley Lab and University of California, Berkeley, scientists has recently discovered a method for controlling the assembly of such complex structures covering a large area from identical helical building blocks, in this case a rod-shaped virus, an accomplishment that could accelerate the development of diverse functional materials.
In nature, a single macromolecule can form diverse functional structures when self-assembled under different conditions. Collagen type I, for example, forms transparent corneal tissues from orthogonally aligned fibers, distinctively colored skin tissues from regularly interspaced fiber bundles, and mineralized tissues from hierarchically organized fibers. However, the origin of tissue assembly is not well understood and nature's self-assembled materials surpass the functional and structural complexity achievable by current fabrication methods. In particular, producing highly ordered structures over a long-range spatial scale has always been a challenge for the artificial self-assembly approach.
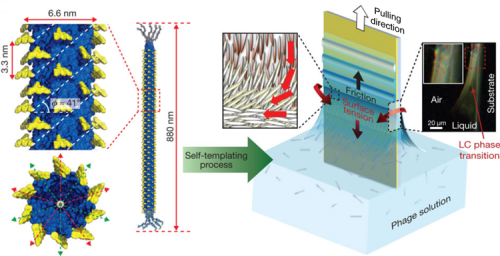
One way to mimic nature is to use liquid crystal, rod-like molecules like collagen that spontaneously align themselves in a flowing environment. The Berkeley scientists used a rod-shaped M13 bacteriophage (a virus that specifically infects bacteria) as the building block, in part because it has structural similarities to collagen. Growth of structures began with immersing a glass substrate in a solution with dispersed phage building blocks and then slowly pulling it out. The researchers precisely controlled the pulling process, so that as the building blocks were carried to the meniscus line of the solution, water evaporated from the exposed surface and the resulting liquid-crystalline structures were imprinted on the substrate.
A single-step process produced supramolecular films with long-range order and multiple levels of hierarchical organization and helical twist. Three distinct supramolecular structures were created by adjusting the concentration of viruses in the solution and the speed with which the glass was pulled: nematic orthogonal twists, cholesteric helical ribbons, and smectic helicoidal nanofilaments. Both liquid-crystalline phase transitions and competing interfacial forces at the meniscus line where the deposition takes place were found to be critical factors in determining the morphology of the imprinted structures during assembly. The resulting materials showed distinctive optical and photonic properties, functioning as chiral reflectors/filters and films with structurally generated color. In addition, M13 phages with genetically incorporated bioactive peptide ligands were able to direct both soft and hard tissue growth in a hierarchically organized manner.
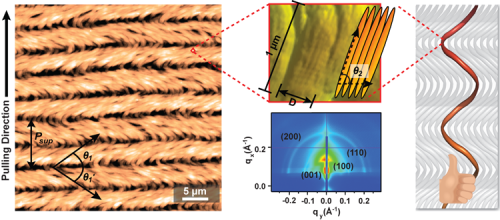
Grazing-incidence small-angle x-ray scattering (GISAXS) measurements conducted on ALS Beamline 7.3.3 revealed the pseudo-hexagonal packed structure of the liquid-crystalline phage films. In addition, the GISAXS spectra of the helicoidal nanofilament structures exhibited significant translational order along the phage particles, as shown by the presence of the (001) diffraction peak. This peak originated from inter-virus positional correlations between the M13 major coat proteins, which corresponded to the axial repeat distance (33 Å) between two adjacent coat proteins.
In sum, the researchers have shown that aspects of nature's self-assembly strategy can be translated to a synthetic system through the controlled growth of phage-based films. Their assembly approach not only provides insight into the complexities of hierarchical assembly in nature but also into how environmental factors control the kinetics during the conversion of helical macromolecules into higher-ordered structures in nature and how these same factors can be used to control assembly in the laboratory. Further development and tuning of synthetic self-templating systems hold promise for the development of advanced structural, optical, and biomedical materials, including sophisticated functional helical-twisted structures.
More information: Publication about this research: W.-J. Chung, J.-W. Oh, K. Kwak, B.Y. Lee, J. Meyer, E. Wang, A. Hexemer, and S.-W. Lee, "Biomimetic self-templating supramolecular structures," Nature 478, 364 (2011).
Journal information: Nature
Provided by Lawrence Berkeley National Laboratory