Beyond stain-resistant: New fabric coating actively shrugs off gunk
Scientists are reporting development and successful testing of a fabric coating that would give new meaning to the phrase "stain-resistant" -- a coating that would take an active role in sloughing off grease, dirt, strong acids and other gunk. The report, which shows that the coating is even more water-repellent than car wax or Teflon, appears in ACS' journal Langmuir.
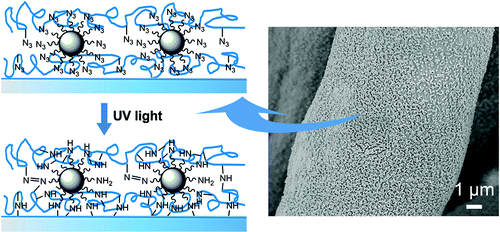
Tong Lin and colleagues explain that a method called "layer-by-layer" (LbL) self-assembly produces films and coatings for sensors, drug-delivery devices and many other products. LbL involves setting down alternate layers of positively and negatively charged materials that are held together by electric charges. With this approach, coatings can be custom-designed for specific applications by selecting the composition of each layer. The downside: These multilayer films are not very stable and eventually come apart. Lin and colleagues wanted to develop a way to stabilize those layers with UV light to form a "superhydrophobic" coating, one that uses natural surface forces to highly repel water and other materials.
Laboratory tests showed that the new coating, applied to cotton fabric, repelled water, acids, bases and organic solvents. The coating also was durable, remaining intact on the cotton fabric after 50 trips through a home washing machine. When the researchers applied several layers of the coating on the fabric, the contact angle (a measure of water-repellence) was about 154 degrees, making it even more repellent than car wax (90-degree contact angle), Teflon (95-degree contact angle) or products that repel rainwater from car windshields (110-degree contact angle).
More information: “Photoreactive Azido-Containing Silica Nanoparticle/Polycation Multilayers: Durable Superhydrophobic Coating on Cotton Fibers” Langmuir, 2012, 28 (15), pp 6328–6335. DOI: 10.1021/la300281q
Abstract
In this study, we report the functionalization of silica nanoparticles with highly photoreactive phenyl azido groups and their utility as a negatively charged building block for layer-by-layer (LbL) electrostatic assembly to produce a stable silica nanoparticle coating. Azido-terminated silica nanoparticles were prepared by the functionalization of bare silica nanoparticles with 3-aminopropyltrimethoxysilane followed by the reaction with 4-azidobenzoic acid. The azido functionalization was confirmed by FTIR and XPS. Poly(allylamine hydrochloride) was also grafted with phenyl azido groups and used as photoreactive polycations for LbL assembly. For the photoreactive silica nanoparticle/polycation multilayers, UV irradiation can induce the covalent cross-linking within the multilayers as well as the anchoring of the multilayer film onto the organic substrate, through azido photochemical reactions including C–H insertion/abstraction reactions with surrounding molecules and dimerization of azido groups. Our results show that the stability of the silica nanoparticle/polycation multilayer film was greatly improved after UV irradiation. Combined with a fluoroalkylsilane post-treatment, the photoreactive LbL multilayers were used as a coating for superhydrophobic modification of cotton fabrics. Herein the LbL assembly method enables us to tailor the number of the coated silica nanoparticles through the assembly cycles. The superhydrophobicity of cotton fabrics was durable against acids, bases, and organic solvents, as well as repeated machine wash. Because of the unique azido photochemistry, the approach used here to anchor silica nanoparticles is applicable to almost any organic substrate.
Journal information: Langmuir
Provided by American Chemical Society


















