Defying conventional wisdom, water can float on oil
Defying thousands of years of conventional wisdom, scientists are reporting that it is possible for water to float on oil, a discovery they say has important potential applications in cleaning up oil spills that threaten seashores and fisheries. Their report appears in ACS' journal Langmuir.
Chi M. Phan and colleagues point out that the ancient Greek philosopher Aristotle made an early attempt to explain flotation around 350 B.C. Today, most people know that less dense liquids float on more dense liquids. So crude oil with a density of about 58 pounds per cubic foot floats on sea water, which has a density of 64 pounds per cubic foot — and not vice-versa. Correct? Phan's team decided to test that notion with computer models and in the lab.
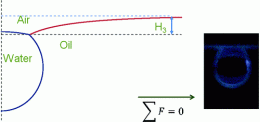
They report that in certain cases, the conventional wisdom is wrong. By adding tiny amounts of water to a floating droplet of oil, they found that the ability of water drops to float at the surface of an oil bath depends on both the size of the droplet and the type of oil. Commercial vegetable oil has enough surface tension – the force between liquid molecules that allows beads of water to form or insects to walk on water -- at its interfaces with air and water to support a droplet's weight, while pure mineral oils do not. At the same time, they found that vegetable oil could not support drops bigger than about one one hundredth of a cubic inch. The authors suggest the new knowledge could help clean up oil spills, where water-borne, oil-eating microbes will mix more easily into the oil if suspended in the tiny droplets they describe. "This result can lead to a new and advanced mechanism in processing oil/water mixtures, such as biodegrading process of unwanted oils, including vegetable oils, sand oil tailings and oil spillages," the authors said.
More information: Can Water Float on Oil? Langmuir, 2012, 28 (10), pp 4609–4613
The floatability of water on oil surface was studied. A numerical model was developed from the Young–Laplace equation on three interfaces (water/oil, water/air, and oil/air) to predict the theoretical equilibration conditions. The model was verified successfully with an oil/water system. The stability of the floating droplet depends on the combination of three interface tensions, oil density, and water droplet volume. For practical purposes, however, the equilibrium contact angle has to be greater than 5° so the water droplet can effectively float. This result has significant applications for biodegrading oil wastes.
Journal information: Langmuir
Provided by American Chemical Society
















