March 16, 2012 report
Graphene battery demonstrated to power an LED

(PhysOrg.com) -- Scientists in Hong Kong have reported, in ArXiv, their experiments to make a graphene battery that they say generates an electrical current by drawing on the ambient thermal energy in the solution in which it is immersed.
Researchers led by Zihan Xu of the Department of Applied Physics and Materials Research Centre at the Hong Kong Polytechnic University, attached silver and gold electrodes to a graphene sheet, typically 7 mm x 7 mm in area, mounted on a silicon substrate. The assembly was then immersed in a saturated solution of copper chloride (CuCl2), and was found to produce an electrical voltage of 0.35 V. They also found that six assemblies arranged in series produced enough electricity to power a light-emitting diode (LED). The device continued to produce around the same voltage for 25 days, but after a month it dropped to about 40 mV.
Graphene is a material that consists of a layer of carbon only one atom thick, and it has been the subject of intense research in recent years because of its unusual properties. One of these properties is an exceptionally high electron mobility.
Xu and colleagues write in their paper that they think the voltage arises from the kinetic energy of the copper ions in the copper chloride solution, which they say is enough to knock electrons out of the graphene, and that these electrons then flow through the sheet. They noted that the voltage increases when the copper chloride solution is heated, and varies with its concentration.
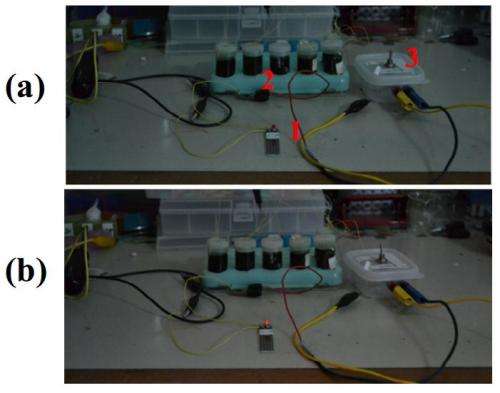
The group also found the voltage increased when the assembly was exposed to pulses of ultrasound, and they say this lends weight to the idea that kinetic energy is the source of the voltage, since the ultrasound would increase the velocity of the copper ions. Small voltages were also produced with ionic solutions such as NaCl and CuSO4. The researchers also carried out control experiments to rule out the possibility that chemical reactions were responsible for the voltage generated.
Dr. Wanlin Guo, the graduate supervisor of one of Xu’s team (Guoan Tai), expressed skepticism at the proposed mechanism, and added that he had so far been unable to reproduce the findings in his own experiments, in which he used graphene sheets of varying sizes, mounted on varying substrates, and with different kinds of electrodes. He was unable to achieve voltages greater than around 0.1 mV.
In 2011, a research group led by Nikhil Koratkar of New York’s Rensselaer Polytechnic Institute also reported on experiments in which graphene was shown to generate a voltage when an ionic solution was made to flow over the sheets. Dr. Guo, of Nanjing University in China, also refuted these results and carried out experiments that showed the interaction of the ions in solution with the electrodes was responsible for the voltage, rather than any interaction with the graphene.
If Xu’s “graphene battery” is harnessing the thermal energy of motion of the ions to generate electricity, this source of energy is essentially unlimited. The researchers say their experimental results provide a “huge breakthrough” in the research into self-powered technology.
More information: Self-Charged Graphene Battery Harvests Electricity from Thermal Energy of the Environment, arXiv:1203.0161v2 [cond-mat.mes-hall] arxiv.org/abs/1203.0161
Abstract
The energy of ionic thermal motion presents universally, which is as high as 4 kJbullet kg-1bullet K-1 in aqueous solution, where thermal velocity of ions is in the order of hundreds of meters per second at room temperature1,2. Moreover, the thermal velocity of ions can be maintained by the external environment, which means it is unlimited. However, little study has been reported on converting the ionic thermal energy into electricity. Here we present a graphene device with asymmetric electrodes configuration to capture such ionic thermal energy and convert it into electricity. An output voltage around 0.35 V was generated when the device was dipped into saturated CuCl2 solution, in which this value lasted over twenty days. A positive correlation between the open-circuit voltage and the temperature, as well as the cation concentration, was observed. Furthermore, we demonstrated that this finding is of practical value by lighting a commercial light-emitting diode up with six of such graphene devices connected in series. This finding provides a new way to understand the behavior of graphene at molecular scale and represents a huge breakthrough for the research of self-powered technology. Moreover, the finding will benefit quite a few applications, such as artificial organs, clean renewable energy and portable electronics.
© 2011 PhysOrg.com


















