Shedding light on how body fends off bacteria
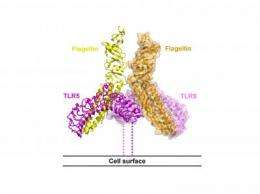
To invade organisms such as humans, bacteria make use of a protein called flagellin, part of a tail-like appendage that helps the bacteria move about. Now, for the first time, a team led by scientists at The Scripps Research Institute and Sanford-Burnham Medical Research Institute has determined the 3D structure of the interaction between this critical bacterial protein and an immune molecule called TLR5, shedding light on how the body protects itself from such foreign invaders.
The study, published February 17 in Science, not only helps decipher the molecular mechanism underlying TLR5 recognition and function, but it also advances knowledge that's key to the design of new therapeutics.
"The structure of the TLR5-flagellin complex visualizes molecular events that occur on the cell surface to trigger flagellin-induced host immune responses, and provides significant insights into the structural basis for TLR5 recognition and signaling," said Ian Wilson, D.Sc., Hansen Professor of Structural Biology at Scripps Research who led the study with Andrei Osterman, Ph.D., professor in Sanford-Burnham's Infectious and Inflammatory Disease Center.
"Gaining knowledge of a molecular interaction and action—as we did in this study— is critically important to the further development of therapeutics based on agonists and antagonists of the TLR5 receptor," said Osterman.
Flagellin is a component in some vaccines and a derivative of this protein is currently being developed as a medical countermeasure to radiation by Cleveland BioLabs, Inc., also a contributor to the new study.
Keeping an eye out for infection
Some of the body's first lines of defense against invading bacteria are Toll-like receptors (TLRs), sensors that sit on the surface of many different types of cells. There are roughly a dozen different TLRs, each keeping an eye out for a particular sign of infection.
TLR5, for example, specifically recognizes and binds to flagellin. Like most TLRs, TLR5 does more than just sense bacteria—it also sends signals that call up immune cells to destroy the intruder. But to fully understand how TLR5 works, scientists needed to be able to see its 3D shape and how it binds to flagellin.
The structures of several other TLRs had already been solved, but each of these binds non-protein molecules, such as RNA or lipids. For technical reasons, determining the structure of TLR5—the only TLR that binds a protein—had long been a challenge.
In this study, the Scripps Research team was able to overcome these hurdles using TLR5 found in zebrafish as a proxy for the human protein. The scientists were then able to apply a technique called X-ray crystallography, which uses powerful X-ray beams to produce 3D images of proteins at the atomic level.
At Sanford-Burnham, Osterman and his team used biochemical and protein engineering methods to unravel the mechanistic details of interactions between TLR5 and flagellin and its derivatives.
Scientists at Roswell Park Cancer Institute and Cleveland BioLabs, Inc. in Buffalo, under the leadership of Andrei Gudkov, Ph.D., performed complementary experiments in human cells expressing TLR5 and validated the fish TLR5 as a good surrogate for human TLR5.
Provided by Sanford-Burnham Medical Research Institute














