Just the two of us: Stable dinucleotide-RNA duplexes show promise in biotechnology
(PhysOrg.com) -- Nucleic acid technology has revolutionized the field of biomedicine, as it can be effectively utilized in the diagnosis, treatment, and prevention of genetic diseases The efficacy of most oligonucleotide therapies is, however, limited as a result of the lability of oligonucleotides in biological fluids and, in particular, their poor delivery to the site of action.
A Swedish team headed by R. Strömberg recently reported in the European Journal of Organic Chemistry that modification of oligonucleotides with a 2'-O-carbamoyl moiety greatly increases the stability of these compounds, which may render their use in constructs for biotechnological and therapeutic applications viable.
Efficiency in the regulation of gene expression is readily achieved if turnover of the target RNA is obtained, but this can only occur if native enzymes recognize the relevant oligonucleotide complex. The ability to catalytically cleave a specific sequence of RNA at a specific site is of high potential value in biotechnology and oligonucleotide therapy. Thus, the development of oligonucleotide-based artificial nucleases (OBANs) as artificial enzymes capable of cleaving mRNA sequences arising from genetic or viral diseases is highly sought.
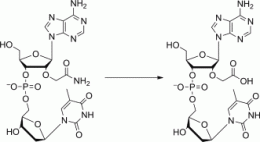
In this context, the scientists set out to modify oligonucleotides with the judicious choice of a 2'-carbamoylmethyl (CM) moiety. Substitution at the 2-position was an important prerequisite, as this has been shown to lead to the formation of stable duplexes with the target RNA, and it was also believed that the CM moiety could further increase the stability of the duplex through hydrogen bonding.
The team was able to show that the 2'-O-carbamoyl modification substantially protected the dinucleotide against enzyme-catalyzed degradation by phosphodiesterase I and made it virtually resistant to degradation by phosphodiesterase II. This, together with the reported increased thermal stability of the duplexes, makes the often-neglected 2'-O-carbamoyl moiety an interesting modification in the pursuit of future compounds that may one day help in the treatment of genetic diseases.
More information: Roger Strömberg, Stability of a 2'-O-(Carbamoylmethyl)adenosine-Containing Dinucleotide, European Journal of Organic Chemistry, dx.doi.org/10.1002/ejoc.201101264
Journal information: European Journal of Organic Chemistry
Provided by Wiley