New insight in how cells' powerhouse divides
New research from the University of California, Davis, and the University of Colorado at Boulder puts an unexpected twist on how mitochondria, the energy-generating structures within cells, divide. The work, which could have implications for a wide range of diseases and conditions, was published today (Sept. 2) in the journal Science.
"It's a paradigm shift in cell biology," said Jodi Nunnari, professor and chair of molecular cell biology at UC Davis and a co-author of the paper.
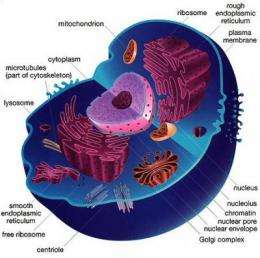
Mitochondria produce chemical energy for a cell's needs. They are wrapped in two membranes, have their own DNA, and can divide to produce new mitochondria. When this division is not properly controlled, it can result in cell death. Defects in mitochondria have been linked to a wide range of degenerative conditions and diseases, including diabetes, cardiovascular disease and stroke.
The research team led by Gia Voeltz, assistant professor in the Department of Molecular Cell and Developmental Biology at CU-Boulder, and Nunnari, at UC Davis, investigated how another structure in the cell, the endoplasmic reticulum or ER, is related to mitochondrial division.
The ER is a complex network of sacs and tubules that spreads out from the nucleus and is distributed throughout the cell. It is thought to play a role in a range of cell processes, including making secretory protein and lipids, and transporting molecules around the cell.
The team found that in both yeast and mammalian cells, mitochondrial division overwhelmingly occurred at points where the two structures, mitochondria and ER, touched.
Previous work by Nunnari's lab and others has shown that mitochondrial division is regulated by dynamin related protein-1, which assembles into a ligature that tightens around the sausagelike mitochondrion and causes it to divide.
Nunnari and Voeltz found that these and other proteins linked to mitochondrial division were also found where the ER and mitochondria touched. Their study indicates that ER tubules first squeeze the mitochondrion, then dynamin-related proteins assemble on the surface to complete the job. This new function for the ER expands and transforms our view of cell organization, Nunnari said.
Provided by University of California - Davis