Heavy metal stars produce Earth-Like planets
New research reveals that, like their giant cousins, rocky planets are more likely to be found orbiting high metallicity stars. Furthermore, these planets are more plentiful around low mass stars. This could have important implications for the search for life outside of Earth.
New research reveals that, like their giant cousins, rocky planets are more likely to be found orbiting high metallicity stars. Furthermore, these planets are more plentiful around low mass stars. This could have important implications for the search for life outside of Earth.
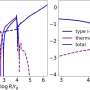
Kevin Schlaufman and Gregory Laughlin, both of the University of California at Santa Cruz, studied the 997 stars with candidate planets thought to be in orbit around them, as reported by Kepler's science team last February. Schlaufman and Laughlin confirmed that both large and small planets were more likely to be found around stars with higher metallicities.
For astronomers, elements other than hydrogen and helium are considered "metals." Stars with high metallicities contain a significant amount of other elements. These metals were first formed when early stars, composed of the two basic gases hydrogen and helium, died in a violent supernova, spewing their contents into space.
Sally Dodson-Robinson, of the University of Texas at Austin, noted that it wasn't surprising to find that terrestrial planets tend to form around more metallic stars.
"Planets formed from the same raw materials as their star does," she explained.

Stars form from the gravitational compression of gas and dust, and the spinning disk of leftover material that orbits the new star is where planets are made.
Before Kepler, enough gas giant planets had been located for astronomers to say with certainty that these behemoths were linked to metal-rich stars. But it was not known if this applied to rocky planets as well, since so few had been found in the galaxy.
That changed in February, when NASA announced the discovery by Kepler of 68 Earth-sized candidates and 288 super-Earths. This planetary goldmine provided a wealth of systems to study, and enough stars to make firmer correlations about the types of stars that less massive planets orbit.
Because all types of planets are more likely to exist around high-metallicity stars, Schlaufman said this gives a rough time frame for when planets first began to appear in the galaxy. After all, they would have to wait for the first generation of stars to speed through their life cycle and explode, providing the metals required for planetary formation. Each cycle of stars would have created more metals, making it easier for planets to coalesce.
The process would have taken a few billion years. This provides constraints on finding advanced civilizations, since planets - and thus life - would not have formed in the early years of the universe.
Schlaufman added that a stronger case could be made as more extrasolar planets are found in the future, helping astronomers better understand the links between planets and their stars.
But in their study, Schlaufman and Laughlin examined more than the metallicity of stars. They also determined that terrestrial planets were more likely to be found around low mass stars.
The reason is simple: gas giants require a lot of mass to form.

"The total mass in the disk is proportional to how massive the star is," Schlaufman said.
Larger disks are more likely to yield massive planets, while smaller stars and their disks seem to result in less massive, rockier satellites.
Schlaufman was quick to note the possibilities for life. Stars more massive than the Sun last only a few billion years, while their lower-mass siblings have much longer lifetimes. This gives a planet more time to develop life - and for that life to evolve into an advanced civilization - before the death of its sun.
The odds of finding life may increase with the more planets that are discovered, especially rocky planets like the Earth. Kepler's confirmation that such planets are more likely to form around high-metal stars should help in this search. Schlaufman points out that Kepler has seven million stars in its field of view, but can only examine about a 160,000 at a time. Although this introduces a bias in the search for new planets, he praises the results Kepler is producing.
Dodson-Robinson agrees.
"If your goal is to find planets, it means you want to look at the most metal-rich stars."
Source: Astrobio.net