'Super sand' for better purification of drinking water (Update)
(PhysOrg.com) -- Scientists have developed a way to transform ordinary sand -- a mainstay filter material used to purify drinking water throughout the world -- into a "super sand" with five times the filtering capacity of regular sand. The new material could be a low-cost boon for developing countries, where more than a billion people lack clean drinking water, according to the report in the ACS journal Applied Materials & Interfaces.
Researchers at Rice University are spinning a bit of nano-based magic to create "coated sand" that has enhanced properties for water purification. The breakthrough may benefit developing countries where more than a billion people lack clean drinking water.
Beds of sand are commonly used throughout the world to filter drinking water. The particle size of sand and surface modifications determine the efficiency of sand in removing contaminants from water.
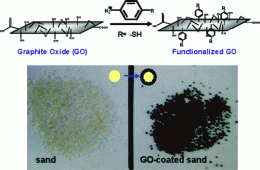
The Rice researchers’ technique makes use of graphite oxide, a product in the chemical exfoliation process of graphite (aka pencil lead) that leads to single-atom sheets known as graphene via subsequent reduction.
A team from the Rice lab of Professor Pulickel Ajayan published a report in the American Chemical Society journal Applied Materials and Interfaces describing a process to coat coarse grains of sand in graphite oxide; the resulting material is several times more efficient at removing contaminants than sand alone.
Nanosheets of graphite oxide can be tailored to have hydrophobic (water-hating) and hydrophilic (water-loving) properties. When mixed in a solution with sand, they self-assemble into coatings around the grains and keep the hydrophilic parts exposed. Adding aromatic thiol molecules to the coatings enhances their ability to sequester water-soluble contaminants.
Ajayan, a Rice professor in mechanical engineering and materials science and of chemistry, and his collaborators from Australia and Georgia conducted experiments to compare this coated sand with plain sand and activated carbon granules used by municipalities and in-home filtration systems.

The researchers ran two model contaminants -- mercury (at 400 parts per billion) and Rhodamine B dye (10 parts per million) -- through sand and coated sand placed into filtration columns. They found coarse sand's adsorption capacity of mercury was saturated within 10 minutes.
The coated sand continued removing mercury for more than 50 minutes and resulted in filtered water with less than one part per billion. (The Environmental Protection Agency's maximum contaminant level goal for mercury in drinking water is two parts per billion.)
Results for water treated with Rhodamine B dye were similar.
The researchers found coated sand sequestered contaminants just as well as the commercially available active carbon filtration systems they tested.
The lab is looking at ways to further functionalize graphite oxide shells to enhance contaminant removal. "By attaching different functional moieties onto graphite oxide, we could engineer some form of a 'super sand' to target specific contaminants species, like arsenic, trichloroethylene and others," said Rice graduate student Wei Gao, primary author of the paper.
More information:
"Engineered Graphite Oxide Materials for Application in Water Purification" ACS Appl. Mater. Interfaces, 2011, 3 (6), pp 1821–1826
DOI: 10.1021/am200300u
Abstract
Retaining the inherent hydrophilic character of GO (graphite-oxide) nanosheets, sp2 domains on GO are covalently modified with thiol groups by diazonium chemistry. The surface modified GO adsorbs 6-fold higher concentration of aqueous mercuric ions than the unmodified GO. “Core–shell” adsorbent granules, readily useable in filtration columns, are synthesized by assembling aqueous GO over sand granules. The nanostructured GO-coated sand retains at least 5-fold higher concentration of heavy metal and organic dye than pure sand. The research results could open avenues for developing low-cost water purification materials for the developing economies.
Provided by American Chemical Society




















