Only the weak survive? Pitt team adds more give for stronger self-healing materials
Conventional rules of survival tend to favor the strongest, but University of Pittsburgh-based researchers recently found that in the emerging world of self-healing materials, it is the somewhat frail that survive.
The team presents in the journal Langmuir a new model laying out the inner workings of self-healing materials made of nanoscale gel particles that can regenerate after taking damage and are being pursued as a coating or composite material. Moreover, the researchers discovered that an ideal amount of weak bonds actually make for an overall stronger material that can withstand more stress.
Although self-healing nanogel materials have already been realized in the lab, the exact mechanical nature and ideal structure had remained unknown, explained Anna Balazs, corresponding author and Distinguished Professor of Chemical Engineering in Pitt's Swanson School of Engineering. The team's findings not only reveal how self-healing nanogel materials work, but also provide a blueprint for creating more resilient designs, she said. Balazs worked with lead author and Pitt postdoctoral researcher Isaac Salib; Chet Gnegy, a Pitt chemical and petroleum engineering sophomore; German Kolmakov, a postdoctoral researcher in Balazs' lab; and Krzysztof Matyjaszewski, a chemistry professor at Carnegie Mellon University with a special appointment in Pitt's Department of Chemical and Petroleum Engineering.
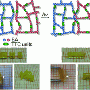
The team worked from a computational model Gnegy, Kolmakov, and Salib created based on a self-healing material Matyjaszewski developed known as nanogel, a composition of spongy, microscopic polymer particles linked to one another by several tentacle-like bonds. The nanogel particles consist of stable bonds—which provide overall strength—and labile bonds, highly reactive bonds that can break and easily reform, that act as shock absorbers.
The computer model allowed the researchers to test the performance of various bond arrangements. The polymers were first laid out in an arrangement similar to that in the nanogel, with the tentacles linked end-to-end by a single strong bond. Simulated stress tests showed, however, that though these bonds could recover from short-lived stress, they could not withstand drawn out tension such as stretching or pulling. Instead, the team found that when particles were joined by several parallel bonds, the nanogel could absorb more stress and still self-repair.
The team then sought the most effective concentration of parallel labile bonds, Balazs said. According to the computational model, even a small number of labile bonds greatly increased resilience. For instance, a sample in which only 30 percent of the bonds were labile—with parallel labile bonds placed in groups of four—could withstand pressure up to 200 percent greater than what could fracture a sample comprised only of stable bonds.
On the other hand, too many labile linkages were so collectively strong that the self-healing ability was cancelled out and the nanogel became brittle, the researchers report.
The Pitt model is corroborated by nature, which engineered the same principle into the famously tough abalone shell, Balazs said. An amalgamation of microscopic ceramic plates and a small percentage of soft protein, the abalone shell absorbs a blow by stretching and sliding rather than shattering.
"What we found is that if a material can easily break and reform, the overall strength is much better," she said. "In short, a little bit of weakness gives a material better mechanical properties. Nature knows this trick."
Provided by University of Pittsburgh