Highway to hydrogenase: A new way to obtain the hydrogenase cofactor azadithiolate
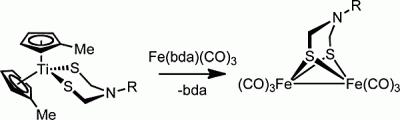
To obtain hydrogen as an energy-rich and environmentally "clean" fuel by an inexpensive, simple method without using expensive metal catalysts preoccupies scientists around the world. Hydrogenases, enzymes employed by organisms to yield hydrogen under anaerobic conditions, are being studied intensively as alternative systems. A very popular research strategy is to build enzyme models that can then be modified to bring them closer to the ultimate goal of functioning even in the presence of some oxygen and not being impeded, or “poisoned”, by the hydrogen gas produced.
Azadithiolate (S−–CH2–NH–CH2–S−) is one of the seven cofactors that make up an important part of such a hydrogenase catalyst. Thomas Rauchfuss and his team at the University of Illinois at Urbana-Champaign developed a new approach to obtain this cofactor, which is described in the Short Communication published in the European Journal of Inorganic Chemistry.
This new approach employs organotitanium compounds, which are known to enable the synthesis of unusual ligands containing sulfur. A dithiolatotitanocene complex was first synthesized, demonstrating that titanocene stabilizes azadithiolate ligands. The next step was to transfer the azadithiolate ligand from the titanocene to a dinuclear iron center, which was successfully carried out with efficiency and good yield.
The importance of this new route to obtain diiron azadithiolato complexes is that it proceeds with high yield and does not require complicated reagents. In addition to describing the first synthesis and structural characterization of an azadithiolato complex not based on the diiron core, the scientists have succeeded in transferring the azadithiolate ligand to the diiron center, which enables further studies of this important cofactor.
More information: Thomas Rauchfuss, A New Route to Azadithiolato Complexes, European Journal of Inorganic Chemistry, dx.doi.org/10.1002/ejic.201001208
Provided by Wiley
















