Synthetic catalyst mimics nature's 'hydrogen economy'
By creating a model of the active site found in a naturally occurring enzyme, chemists at the University of Illinois have described a catalyst that acts like nature's most pervasive hydrogen processor.
The researchers describe their work in a paper accepted for publication in the Journal of the American Chemical Society, and posted on the journal's Web site.
Scientists have long been puzzled by nature's ability to use cheap and plentiful building blocks - iron, nickel and sulfur - to achieve the catalytic performance seen in rare and expensive metals. In particular, two enzymes - iron-iron hydrogenase and nickel-iron hydrogenase - function as hydrogen processors, much like platinum.
"Nature relies on a very elaborate architecture to support its own 'hydrogen economy,' " said Thomas B. Rauchfuss, a professor of chemistry and corresponding author of the paper. "We cracked that design by generating mock-ups of the catalytic site to include the substrate hydrogen atom."
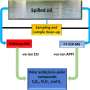
The researchers' model of the nickel-iron complex is the first to include a bridging hydride ligand, an essential component of the catalyst.
"By better understanding the mechanism in the nickel-iron hydrogenase active site, we are learning how to develop new kinds of synthetic catalysts that may be useful in other applications," said graduate student Bryan E. Barton, lead author of the paper.
"The study of hydrogenases offers plenty of potential glamour - such as the hydrogen economy, green energy and bio-fuel cells - but the lasting breakthroughs result from manipulable mechanistic models like ours," said graduate student and co-author Matthew Whaley. "By building a model that contains a hydride ligand, we have proven that the behavior of these natural catalysts can be understood and optimized."
Source: University of Illinois at Urbana-Champaign (news : web)