Electron gas on insulator's surface opens way to multifunctional transistors
French researchers have succeeded in creating a conductive layer on the surface of strontium titanate (SrTiO3), a transparent insulating material considered to be very promising for the development of future microelectronics applications. Two nanometers thick, this conductive layer is a two-dimensional metallic electron gas (2DEG) that is part of the insulating material. Easy to produce, it opens new possibilities for electronics based on transition metal oxides (the SrTiO3 family), taking advantage of these materials' vast range of physical properties (superconductivity, magnetism, thermoelectricity, etc.) to integrate a number of different functions in a single microelectronic device. A paper explaining this unexpected discovery, arising from research at the SOLEIL synchrotron, was published in the January 13, 2011 issue of Nature magazine.
Today's microelectronic components consist of layers of semiconductors on a silicon substrate. In order to sustain the pace of periodic upgrades in the performance of microelectronic devices beyond 2020, alternative technological solutions are being investigated. Researchers are increasingly turning their attention to transition metal oxides , which offer promising physical properties such as superconductivity, magnetoresistance, thermoelectricity, multiferroicity and photocatalytic capacity.
Within this family of materials, strontium titanate (SrTiO3) has been the subject of extensive research. This insulating material becomes a good conductor when it is doped, for example by creating a few surface oxygen vacancies. The interfaces between SrTiO3 and other oxides (LaTiO3 or LaAlO3) are conductive, even though the two materials are insulators. Moreover, they offer properties like superconductivity, magnetoresistance and thermoelectricity, with very good performances at room temperature. The problem, however, is that interfaces between oxides are very difficult to produce.
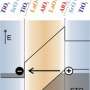
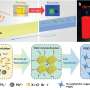
Now an unexpected discovery has burst through this technological barrier. An international team led by researchers at CNRS and Université Paris-Sud 11 has produced a two-dimensional metallic electron gas (2DEG) on the surface of SrTiO3. This conductive layer, approximately two nanometers thick, was obtained by vacuum-cleaving a piece of strontium titanate, a very simple and economical process. The constituent elements of SrTiO3 are natural resources available in large quantities, and the compound is non-toxic, unlike the materials most widely used in microelectronics today (bismuth tellurides). In addition, 2DEGs could probably be created on the surface of other transition metal oxides using a similar technique.
The discovery of a conductive layer of this type (not requiring the addition of a layer of another material) is a significant step forward for oxide-based microelectronics. It could make it possible to combine the intrinsic multifunctional properties of transition metal oxides with those of the two-dimensional metal on their surface. Possible developments could include the coupling of a ferroelectric oxide with the electron gas on its surface to produce non-volatile memories, or the inclusion of transparent circuits on the surface of solar cells or touch screens.
The 2DEG on the surface of strontium titanate was identified and studied in experiments using angle-resolved photoemission spectroscopy (ARPES) at the SOLEIL synchrotron in Saint-Aubin, France, and the Synchrotron Radiation Center at the University of Wisconsin, USA.
More information: Two-dimensional electron gas with universal subbands at the surface of SrTiO3, A. F. Santander-Syro, O. Copie, T. Kondo et al., Nature, 13 January 2011.
Provided by CNRS