Research show oysters could help clean up the reef through filtration
Scientists have found oysters could be very useful in gobbling up nutrient pollution from tropical waterways, including the Great Barrier Reef.

Scientists have found oysters could be very useful in gobbling up nutrient pollution from tropical waterways, including the Great Barrier Reef.
Ecology
Apr 24, 2024
0
68

Much like how electric circuits use components to control electronic signals, quantum networks rely on special components and nodes to transfer quantum information between different points, forming the foundation for building ...
Optics & Photonics
Apr 24, 2024
0
17

Excessive nitrogen input into agriculture systems has caused environmental problems such as atmospheric pollution, loss of biodiversity and degradation of water. Meanwhile, the development of intensive animal farming has ...
Agriculture
Apr 22, 2024
0
3

Nitrogen fertilizers play an essential role in ensuring global food security. However, the applied fertilizer-nitrogen, particularly that exceeding crop demand and soil N retention capacity, can potentially escape into the ...
Ecology
Apr 16, 2024
0
1

Researchers led by Satoshi Kamiguchi at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan have discovered a greener way to produce ammonia, an essential compound used in fertilizers.
Analytical Chemistry
Apr 15, 2024
0
151

Ring-shaped chemical structures called saturated heterocycles are found in most FDA-approved drugs but are often difficult to create. Scripps Research chemists have just developed a surprisingly easy method for making many ...
Materials Science
Apr 13, 2024
0
85

Assessing the function of forest ecosystems requires a deep understanding of the mechanisms of soil nitrogen mineralization. A study conducted by a team of researchers has shed light on how soil N-cycling genes drive soil ...
Ecology
Apr 12, 2024
0
9

Insect pheromones are odor molecules used for chemical communication within a species. Sex pheromones play a crucial role in the mating of many insects. Species-specific odors attract males and females of the same species. ...
Ecology
Apr 11, 2024
0
2

Sunlight has a major influence on chemical processes. Its high-energy UV radiation in particular is strongly absorbed by all materials and triggers photochemical reactions of the substances present in the air. A well-known ...
Optics & Photonics
Apr 10, 2024
0
31
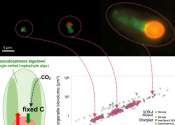
Nitrogen is a nutrient essential for all life on Earth. Although nitrogen gas (N2) is plentiful, it is largely unavailable to most organisms without a process known as nitrogen fixation, which converts dinitrogen to ammonium—a ...
Evolution
Apr 3, 2024
0
319
Nitrogen (pronounced /ˈnaɪtrədʒɨn/) is a chemical element that has the symbol N and atomic number 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless and mostly inert diatomic gas at standard conditions, constituting 78% by volume of Earth's atmosphere.
Many industrially important compounds, such as ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, contain nitrogen. The extremely strong bond in elemental nitrogen dominates nitrogen chemistry, causing difficulty for both organisms and industry in converting the N2 into useful compounds, and releasing large amounts of energy when these compounds burn or decay back into nitrogen gas.
The element nitrogen was discovered by Daniel Rutherford, a Scottish physician, in 1772. Nitrogen occurs in all living organisms. It is a constituent element of amino acids and thus of proteins, and of nucleic acids (DNA and RNA). It resides in the chemical structure of almost all neurotransmitters, and is a defining component of alkaloids, biological molecules produced by many organisms.
This text uses material from Wikipedia, licensed under CC BY-SA