This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Why do materials get stronger when they are deformed? Research sheds light on universal mechanisms of work hardening
The earliest blacksmiths in the Bronze and Iron Ages figured out that when they deformed metal through bending or hammering, it became stronger. This process, known as work or strain hardening, is still used widely in metallurgy and manufacturing today to increase the strength of everything from car frames to overhead power wires. But materials scientists have never been able to watch this essential process unfold in real time—until now.
A team of scientists from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have observed, for the first time, the detailed mechanisms driving the fundamental process of work hardening.
The research, conducted at the Harvard Materials Research Science and Engineering Center (MRSEC), provides a deeper understanding of material strength, which could have a wide-ranging impact on material design and manufacturing.
The findings are published in the journal Nature.
"Work hardening is used in many industrial deformation processes," said Frans Spaepen, John C. and Helen F. Franklin Professor of Applied Physics at SEAS and senior author of the study. "Today, large-scale computer programs are used to model work hardening, but if you want to make those models as effective as possible, we need to know more about the underlying laws that govern that process. This work gives us a real-time window into the universal process of work hardening."
It's been impossible to observe work hardening in metals in real time because the atomic structures can only be observed through an electron microscope. Researchers can compare the structure before and after deformation but have had only a limited view into what happens during the process. Previous research has revealed that imperfections in the structure, known as dislocations, form a network of defects which cause the work hardening.
"What hasn't been clear is the full complexity of the interactions between the defects in these atomic crystals that lead to hardening," said Ilya Svetlizky, a former SEAS postdoctoral fellow with the MRSEC and co-first author of the paper.
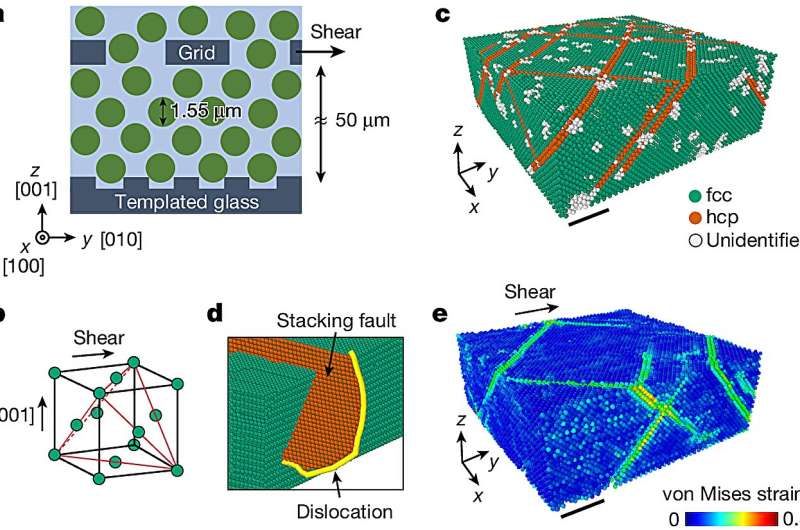
To understand that critical part of the process, the research team turned to colloidal crystals—particles that are about 10,000 times larger than atoms and spontaneously form a crystal structure at high concentrations. These crystals are used to mimic atomic systems because they have the same structures, undergo the same phase transitions, and possess the same types of defects. Colloidal crystals, however, are very soft—even 100,000 times softer than Jell-O.
The researchers grew these colloidal crystals composed of millions of particles and observed each particle using a confocal optical microscope. When they applied a strain to these crystals, they could measure the motion of each and every particle.
Surprisingly, these colloidal crystals experience significant work hardening—even more strongly than any other material. In fact, when the difference in particle size is taken into account, these ultra-soft materials become much stronger than most metals.
"We did not expect that hard-sphere colloidal crystals could be work hardened," said Seongsoo Kim, a MRSEC supported graduate student of Spaepen and Weitz and co-first author of the paper. "The interactions between particles are too simple compared to normal metals. In fact, we found that these soft materials exhibit exceptionally pronounced work hardening, even more so than most metals like copper and aluminum."
It is the first time that work hardening has been observed in colloidal crystals; it reveals that the process is governed primarily by the geometry of the particles and the defects. The crystals became stronger because of the dislocation defects, how they interact and entangle with one another.
These observations reveal the universal mechanisms of work hardening which will also apply more generally to all materials, even those that cannot be studied with optical microscopes. These soft colloidal crystals exhibit such exceptionally large work hardening because they can contain a very high density of these defects.
"This research tells us something fundamental and universal about how materials get stronger. These are amazing materials: Even though they are very soft, work hardening makes them into the strongest materials known," said David A. Weitz, Mallinckrodt Professor of Physics and of Applied Physics, and a co-author of the paper.
More information: Seongsoo Kim et al, Work hardening in colloidal crystals, Nature (2024). DOI: 10.1038/s41586-024-07453-6
Journal information: Nature