This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Novel glass-forming liquid electrolyte shows glass transition across broad range

As the world shifts towards a more sustainable future, the development of advanced electrochemical devices, such as rechargeable batteries with higher energy densities and efficient electrodeposition capabilities, has become increasingly crucial. In recent years, ultra-concentrated electrolyte solutions, where metal salts are dissolved at concentrations two to three times higher than those in a single solvent, or mixtures where metal salts are excessively dissolved in a single solvent, have gained attention as new electrolyte solutions.
These solutions remain liquid at room temperature and enable high ion conduction and high-efficiency, high-quality metal film formation. However, the physicochemical or thermodynamic definition of these liquids remains unclear. Moreover, identifying the dissolved species and understanding their structures, which are crucial for their use as electrolytes, is extremely challenging.
A research team from Niigata University, led by Prof. Yasuhiro Umebayashi and Dr. Jihae Han, along with Dr. Hikari Watanabe from Tokyo University of Science, from a solution chemistry perspective, has been studying the mechanisms of specific lithium-ion conduction in lithium solvate ionic liquids and highly concentrated electrolyte solutions. They found a novel glass-forming liquid electrolyte, a two-component mixture of cyclic sulfone and lithium salt, which exhibits a glass transition across a broad compositional range.
Furthermore, to elucidate the uniquely high Li+ transference number in these mixtures, speciation and dipole reorientation dynamics were studied to provide evidence of large-size aggregate formation in these mixtures. These findings have been published in the Faraday Discussions on 10 June 2024.
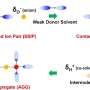
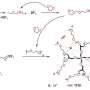
The thermophysical properties of both lithium salt-1,3-propanesultone (PS) and lithium salt-sulfolane (SL) binary mixtures showed that only glass transition was observed in a certain lithium salt concentration range. Raman spectroscopy revealed that lithium ions exist in solution as contact ion pairs (CIPs) and aggregates (AGG) in solution.
In addition, two-dimensional correlation analysis of Raman spectra and dielectric relaxation spectra (DRS) successfully attributed the observed relaxation in DRS. It suggests that AGGs generated at high lithium salt concentration have a large spatial scale and play an important role in the specific lithium-ion conduction.
To achieve the Sustainable Development Goals (SDGs) and the objectives of Society-5, there is a growing demand for next-generation energy storage devices that can store electric energy efficiently and are tailored for specific applications. The development of these devices, utilizing both liquid and solid electrolytes, has become more advanced.
"Our research into glass-formed liquid electrolytes marks a significant leap towards bridging the gap between traditional liquid and solid electrolytes," explains Professor Yasuhiro Umebayashi, the corresponding author. "These materials offer unique advantages in terms of efficiency and application-specific adaptability, paving the way for next-generation energy storage devices."
More information: Yasuhiro Umebayashi et al, Speciation and dipole reorientation dynamics of glass-forming liquid electrolytes: Li[N(SO2CF3)2] mixtures of 1,3-propane sultone or tetrahydrothiophene-1,1-dioxide, Faraday Discussions (2024). DOI: 10.1039/D4FD00050A
Provided by Niigata University