This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists discover molecular mechanism that plays key role in gene transcription and macrophage functional activation

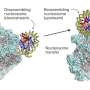
As the largest variant of the histone H2A family, macroH2A plays significant regulatory roles in various processes such as X-chromosome inactivation, embryonic development, cellular metabolism, and tumorigenesis due to its unique linker and macro domains.
The histone chaperone Facilitates Chromatin Transcription (FACT) can clear macroH2A from specific locations on the chromatin, thereby activating gene transcription. However, the core mechanism by which macroH2A regulates gene expression through FACT still requires further elucidation.
A collaborative study by Prof. Li Wei from the Institute of Biophysics of the Chinese Academy of Sciences and Prof. Chen Ping from the Capital Medical University has unveiled for the first time the molecular mechanism by which the histone variant macroH2A regulates the nucleosome maintenance function of FACT through the critical S139 site.
The findings are published in the journal Molecular Cell.
The study determined that this mechanism plays a key role in gene transcription activation and macrophage functional activation.
The researchers employed an in vitro nucleosome assembly system and combined it with single-nucleosome magnetic tweezers of high spatiotemporal resolution they developed independently. With this, they then analyzed the effects of the macro and linker domains of macroH2A on nucleosome stability and structural dynamics.

Results showed that macroH2A promotes FACT binding, significantly reduces nucleosome stability, and mediates the depletion of macroH2A from nucleosomes.
Further research revealed that the S139 of macroH2A serves as a key site that regulates the interaction between FACT and macroH2A. In macrophages, mutating the S139 of macroH2A can reshape its localization on the chromatin and alter gene expression levels within the macrophages.
This study combined various technical methods to reveal the crucial role of FACT in mediating the clearance of macroH2A from nucleosomes and ultimately identified the S139 in the macroH2A linker region as an important site regulating the interaction between the FACT complex and macroH2A.
This work clarifies for the first time the interaction mechanism between the FACT complex and the variant macroH2A at the molecular level. The study provides a theoretical foundation for deeper understanding of their interaction in gene transcription regulation. It also offers insights into the roles of macroH2A and FACT in regulating macrophage functions.
More information: Dengyu Ji et al, FACT mediates the depletion of macroH2A1.2 to expedite gene transcription, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.07.011
Journal information: Molecular Cell
Provided by Chinese Academy of Sciences