This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Practical formate/bicarbonate energy system shows promise for hydrogen storage

Researchers are still looking for an ideal way to safely and stably store hydrogen, the beacon of hope for the energy transition. Researchers from the Leibniz Institute for Catalysis in Rostock, LIKAT, and the company H2APEX report on how this volatile and combustible gas can be tamed safely and with simple "ingredients." The study is published in Nature Communications.
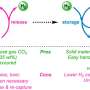
Together, they developed a homogeneous catalyst system with which they can bind hydrogen (H2) to potassium bicarbonate and thus store it chemically in a safe and stable manner. Bicarbonate is a salt of carbonic acid, commonly known as baking powder or baking soda.
The hydrogen reacts with bicarbonate in the described system in the presence of a ruthenium catalyst to formate, an equally harmless salt, namely that of formic acid. "We can release the hydrogen stored in the formate again at any time—with the same catalyst, in the same system," explains Dr. Rui Sang and Ph.D. student Carolin Stein, both first authors of the scientific publication. Such a reaction, which can proceed in either direction, is called reversible.
According to research group leader Dr. Henrik Junge, the system works stably at temperatures of about 60°C. The reaction takes place in a solution containing all the chemical substances involved: Hydrogen and bicarbonate as well as the catalyst, which makes the reaction possible in the first place and is not consumed in the process itself.
In the process described in this latest publication, it is based on ruthenium, which is commercially available. In the end, the solution also contains the newly formed formate—the actual H2 store.
The system is also easy to control technically, says Dr. Sponholz, Head of Research at H2APEX: "Depending on the pressure at which I add the hydrogen to the system, the gas is either bound to the bicarbonate to form formate or the reaction is reversed and the formate releases the hydrogen again."
Easy to store and transport
Hydrogen plays a key role in alternative energy supply scenarios. And methanol, ammonia and methane are being discussed as storage media for a future hydrogen economy. Formic acid salts have an advantage over these storage media in terms of the toxicity of the substances and energy consumption. Formate could be easily stored in plastic containers and transported in tankers. Henrik Junge says, "Basically like milk, beer or diesel."
Together with bicarbonate, the formate forms an energy system that is charged or discharged via hydrogen like a battery. Such a system is actually suitable for use in local, rural areas in particular. There, wind power or solar energy can produce green hydrogen via electrolysis during phases when more electricity is supplied than is consumed, which is then stored as formate.
In the cooperation between LIKAT and H2APEX, one of the researchers' aims is to store as much hydrogen as possible in the formate. This is influenced by the storage density, solubility and molarity of the salt used, properties which in turn depend on its counter ion. This is because salts usually consist of ions of opposite charge, the cation and the anion.
After testing several candidates and weighing up the pros and cons, the decision was made to use potassium, says Dr. Peter Sponholz. The salt that is charged with hydrogen in the battery is therefore precisely called potassium bicarbonate. By the way: baking powder for the kitchen usually contains sodium bicarbonate.
40 cycles for a climate-neutral process
The authors emphasize that the process is CO2 -neutral. Normally, when hydrogen is recovered, some of the bicarbonate is decomposed into CO2 and released, explains Carolin Stein. "Our system, on the other hand, holds the CO2 permanently." This means that pure hydrogen can be obtained from this storage system, which can be used directly in a fuel cell without further purification.
In their paper, the authors report 40 consecutive cycles of hydrogen storage and release over a period of six months. Using minimal amounts of the ruthenium catalyst in the ppm range, the chemists produced 50 liters of hydrogen with an average purity of 99.5% with their laboratory system.
The company H2APEX in Rostock-Laage is using these results, among other things, to build a larger demonstrator, for which the industrial partner is also using the institute's technical center, Catalysis2Scale. If everything goes as planned, the corresponding plant will be commercialized by the end of 2025, and the chemical symbol for hydrogen atoms, H, will then also mean H for hope for the energy transition.
More information: Rui Sang et al, Development of a practical formate/bicarbonate energy system, Nature Communications (2024). DOI: 10.1038/s41467-024-51658-2
Journal information: Nature Communications
Provided by Leibniz Institute for Catalysis