This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Enhanced model enables more realistic biofilms for studying ventilator pneumonia
Scientists at The University of Warwick have made a breakthrough which could help find new treatments for a deadly infection that can affect up to 40% of hospital patients using mechanical ventilators. The study is published in Microbiology.
Ventilator-associated pneumonia (VAP) is a common infection in patients using ventilators, particularly for those with existing respiratory conditions like COVID-19.
VAP is transmitted by germs that stick to the breathing tubes, which are often resistant to antibiotics. Up to 40% of ventilated patients in intensive care wards will develop VAP, with 10% of those patients dying as a result.
In a study, published in Microbiology, researchers recreated hospital conditions to improve understanding of the infection.
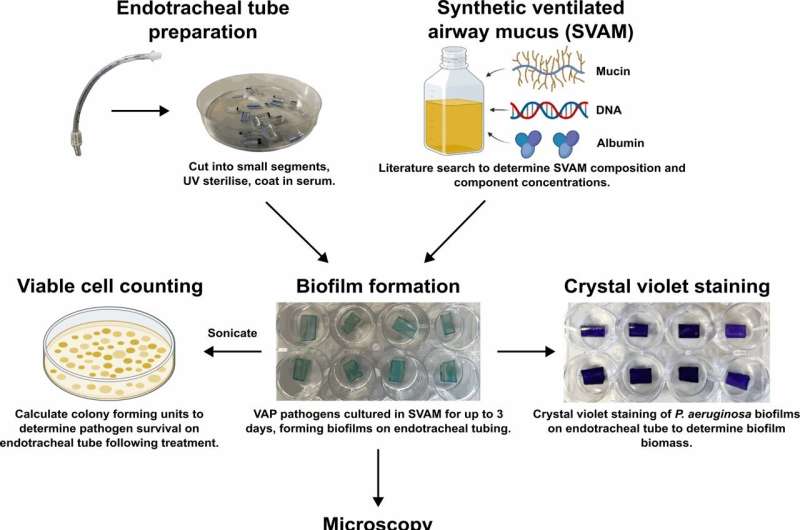
They used the same type of tubes that go into patients' airways and created a special mucus to simulate the conditions inside a human body. Bacteria and fungi formed a slimy layer called a biofilm on these tubes.
Dr. Dean Walsh, Research Fellow, University of Warwick, said, "Our study found that the biofilms in our model were different and more complex than those usually grown in standard lab conditions, making them more realistic.
"The biofilms formed in this new model were very tough to get rid of, even with strong antibiotics, much like what happens in real patients.
"Significantly, when we combined antibiotics with enzymes that break down the biofilm's protective slime layer, the biofilms were more successfully removed than with antibiotics alone. With the enzymes, we could halve the concentration of antibiotics needed to kill the biofilms. So, that suggests we can use our model to identify new VAP treatments that attack the slime layer."
Dr. Freya Harrison, School of Life Sciences, University of Warwick, added, "VAP is a killer, and there are currently no cost-effective ways of making the tubes harder for microbes to colonize. Our new model can help scientists develop better therapies and design special tubes that prevent biofilms, which could improve the health of patients on ventilators."
This project was part of an international research program in antimicrobial resistance that brings together colleagues at the University of Warwick with those at Monash University in Melbourne and is supported by the Monash-Warwick Alliance.
Professor Ana Traven, co-Director of the Monash-Warwick Alliance program in emerging superbug threats, and co-author of the study, added, "It is exciting that we could join forces with our colleagues at Warwick for this important study. Many promising new anti-infectives fail because experiments done in the laboratory do not recapitulate very well the more complex infections that occur in patients.
"As such, the development of laboratory models that mimic disease, such as was done in this study, is important for accelerating the discovery of credible antimicrobial therapies that have a higher chance of clinical success."
More information: Dean Walsh et al, A new model of endotracheal tube biofilm identifies combinations of matrix-degrading enzymes and antimicrobials able to eradicate biofilms of pathogens that cause ventilator-associated pneumonia, Microbiology (2024). DOI: 10.1099/mic.0.001480
Provided by University of Warwick