This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unique mechanism protects pancreatic cells from inflammation in mice
Researchers from the University of Cologne have revealed a mechanism protecting pancreatic β-cells, which are crucial for insulin production from inflammatory cell death. The study investigated the role of receptor-interacting protein kinase 1 (RIPK1) in regulating β-cell survival.
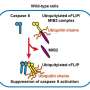
Typically, this protein controls cell fate by balancing survival and death signals in response to inflammatory cytokines like tumor necrosis factor (TNF). However, the team of Dr. Nieves Peltzer at the Center for Molecular Medicine Cologne (CMMC) found out that RIPK1 is not essential for β-cell survival under both normal and diabetic conditions. The authors suggest that the exceptionally high levels of the protective molecule cFLIP expressed by β-cells might be responsible for protecting them from the RIPK1 checkpoint.
The study "RIPK1 is dispensable for cell death regulation in β-cells during hyperglycemia" was published in Molecular Metabolism.
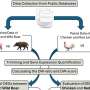
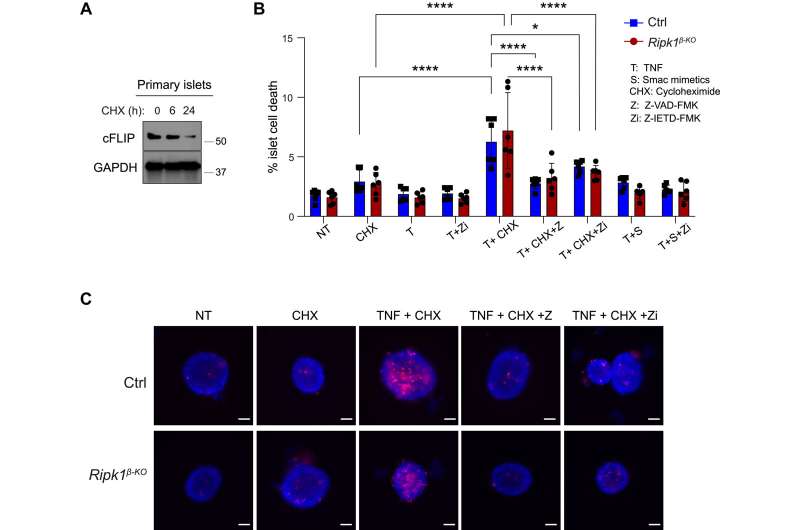
Using a mouse model, the researchers discovered that β-cells exhibit high levels of the anti-apoptotic protein cFLIP, which prevents cell death, and low levels of apoptotic (caspase-8) and necroptotic (RIPK3) proteins, which promote cell death, providing a protective shield against inflammatory TNF-induced cell death. Treatment with the antibiotic cycloheximide, which reduces cFLIP levels, made the pancreatic islets sensitive to TNF-induced cell death, underscoring the central role of protein expression and cFLIP levels in this protective mechanism.
"Our findings suggest that pancreatic β-cells possess a distinct protective mechanism against TNF-induced cytotoxicity, reliant on cFLIP but not on RIPK1. This could lead to novel strategies for preserving β-cell function in diabetic patients," said Peltzer.
These results challenge the dominant paradigm that RIPK1 is universally required for cell death regulation across all cell types. Instead, pancreatic β-cells appear to be uniquely able to resist inflammatory death signaling—a discovery that breaks new grounds for diabetes research.
Önay Veli, first author of the study, added, "We were amazed by the remarkable resistance of β-cells to TNF-induced cell death. We found that β-cells express elevated levels of pro-survival proteins compared to pro-death proteins, which helps us to better understand their resistance mechanism."
In the future, it would be important to explore in details the mechanism by which cFLIP regulate β-cell survival during diabetes, with a focus on therapeutic options to protect β-cells from immune attack or glucotoxicity. In addition, future research may lead to the discovery of strategies to improve β-cell viability during transplantation.
More information: Önay Veli et al, RIPK1 is dispensable for cell death regulation in β-cells during hyperglycemia, Molecular Metabolism (2024). DOI: 10.1016/j.molmet.2024.101988
Journal information: Molecular Metabolism
Provided by University of Cologne