This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Catalysts with single non-noble metal component for efficient conversion of biomass-based chemicals
Utilization of biomass as the basic feedstock for the production and chemicals and energy storage has been demonstrated to be an important alternative to achieve sustainable society, which has attracted increasing interests in both academic and industrial communities for decades.
5-hydroxymethylfurfural (HMF), one of the most important bio-based platform compounds, could serve as a bridge feedstock between biomass resources and chemicals. It is possible to synthesize a series of high-value added chemicals from HMF through hydrogenation, oxidative dehydrogenation, esterification, hydrolysis, etc., due to the presence of both aldehyde and hydroxymethyl groups.
Among them, the selective hydrogenolysis of HMF to 2,5-dimethylfuran (DMF) as a potential liquid biofuel candidate has attracted extensive attention.
As a biomass fuel, DMF can be used in internal combustion engines with higher compression ratios to improve fuel utilization efficiency as a result of its higher RON (Research octane number, 119) compared to those of commercial gasoline (90–100) and ethanol (110).
In addition, DMF could be the important reactant for the direct synthesis of bio-based p-xylene (PX) by Diels-Alder reaction with ethylene. Compared to the conventional petroleum-based PX production process, such bio-based process shows high selectivity and avoids the complex separation procedure.
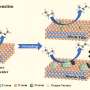
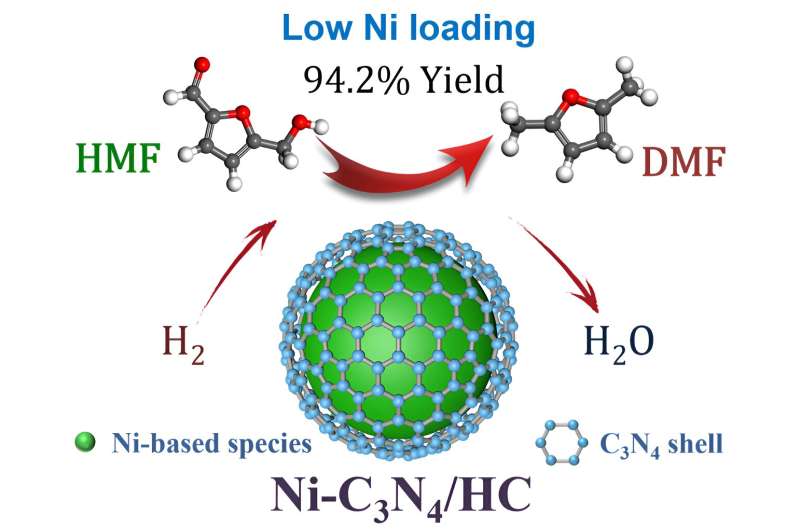
A research team led by Prof. Weimin Yang from East China University of Science and Technology and Sinopec Shanghai Research Institute of Petrochemical Technology Co., Ltd. report that the Ni-C3N4 catalyst supported on H2 activated carbon (HC) with ultra-low Ni loading for the selective hydrogenolysis of HMF to DMF.
The research is published in the Chinese Journal of Catalysis.
The Ni-C3N4/HC catalyst achieves DMF yield of 94.2% with high sustainability (longer than 120 h life time in fixed-bed reactor), and exhibits remarkably high productivity (12.8 mmolDMF⋅mmolNi-1 ⋅h-1) across the temperatures of 100–200°C, outperforming the recently reported state-of-the-art Ni, Co and Cu-based catalysts, and is also comparable to noble metal catalysts (Ru, Pd and Pt).
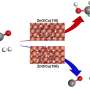
The combination of characterizations and theoretical calculations revealed that Ni3N is the active component for the hydrogenolysis, and the C3N4 shells stabilize the Ni particles and prevent the agglomeration during the reaction.
This work could advance the design of single non-noble metal catalysts for hydrogenation and hydrogenolysis reactions.
More information: Hongyu Qu et al, Efficient hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Ni-C3N4 catalysts with ultra-low Ni loading, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(24)60017-3
Provided by Chinese Academy of Sciences