This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Toward non-toxic antifouling agents: A novel method for total synthesis of scabrolide F
Marine organisms produce many organic compounds with diverse chemical structures and biological activities. These natural marine products are regarded as potential starting points for the discovery and development of new drugs.
Among these are norcembranolide diterpenes isolated from the soft corals of the genus Sinularia. These compounds exhibit diverse biological activities, and many of them have anti-cancer and anti-inflammatory properties.
Consequently, many studies have investigated the properties of norcembranolide diterpenes and their synthesis methods. Given their potential in drug discovery, developing a synthetic method to produce these compounds is essential. However, reports on the total synthesis of norcembranolide diterpenes are limited.
In a recent study, a team of researchers from Japan led by Associate Professor Hiroyoshi Takamura and including Yuki Sugitani, Ryohei Morishita, and Isao Kadota, all from the Department of Chemistry, Graduate School of Natural Science and Technology at Okayama University, achieved the total synthesis of a norcembranolide diterpene.
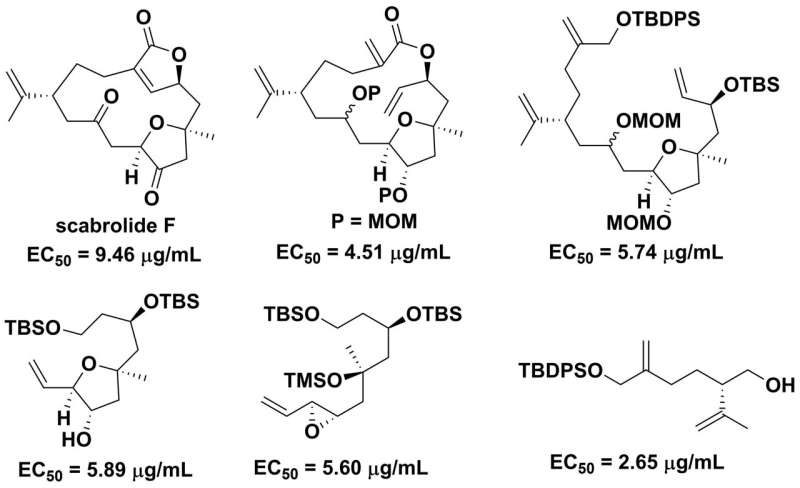
"In this research, we succeeded in the chemical synthesis of scabrolide F, a natural product that was isolated from a soft coral Sinularia scabra.
"Moreover, we found that synthetic scabrolide F and its related compounds exhibited antifouling activity without toxicity, which can potentially prevent the damage caused by biofouling, namely, the accumulation of microorganisms, plants, and algae," explains Dr. Takamura.
The study, published in the journal Organic & Biomolecular Chemistry on May 23, 2024, included contributions from Takefumi Yorisue from the Institute of Natural and Environmental Sciences, University of Hyogo, as well as the Division of Nature and Environmental Management, Museum of Nature and Human Activities.
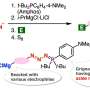
To synthesize scabrolide F, the researchers designed a hydrocarboxylic acid compound as the key intermediate. They initially attempted to synthesize the intermediate through the coupling of the tetrahydrofuran and the dithiane fragments. The coupling product could then be transformed into the intermediate through an allylation reaction.
However, their model study for testing the allylation reaction failed to yield the desired product, prompting the team to pursue an alternative method.
In the second approach, they successfully fabricated the intermediate through a reaction called fragment coupling between an alkyl iodide and an aldehyde. The intermediate was then transformed to the desired scabrolide F, through two key transformations, namely, macrolactonization, which refers to the intramolecular reaction between the hydroxyl group and carboxylic group of the intermediate, and transannular ring-closing metathesis, which forms a ring within an existing macrocyclic ring.
This innovative method represents the total synthesis of scabrolide F.
Furthermore, the researchers evaluated the antifouling activity and toxicity of this compound and its five key synthetic intermediates against the cypris larvae of the barnacle Amphibalanus amphitrite.
All six compounds were found to have antifouling activity with 50% effective concentration or EC50 values ranging from 2.65–9.46 μg/mL and were non-toxic with 50% lethal concentration or LC50 toxicity level greater than 50 μg/mL. Additionally, one of the intermediates was identified as suitable for the preparation of non-toxic antifouling agents.
The synthesis method present in the study will also be useful for the synthesis of other norcembranolide diterpenes. Dr. Takamura says, "Preventing damage caused by biofouling is a key global issue. It is expected that organic compounds synthesized in this research can be used to create new antifouling agents and paints."
Overall, the strategy for the total synthesis of norcembranolide diterpenes proposed in this study can aid in the development of antifouling agents and the discovery of new drugs.
More information: Hiroyoshi Takamura et al, Total synthesis and structure–antifouling activity relationship of scabrolide F, Organic & Biomolecular Chemistry (2024). DOI: 10.1039/D4OB00698D
Provided by Okayama University