This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking RNA functionality: A redox-responsive approach
National University of Singapore (NUS) chemists have developed a strategy using disulfide-containing small molecules to facilitate the reversible control and delivery of ribonucleic acid (RNA).
RNA-based therapeutics have emerged as one of the most sought-after therapeutic modalities in recent years. However, RNA delivery remains a major challenge in the field. Lipid nanoparticles, despite being widely used for RNA delivery including the delivery of COVID-19 mRNA vaccines, face several limitations such as their effectiveness and safety. Alternative methods that can potentially overcome these limitations are highly desirable.
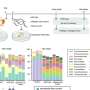
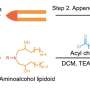
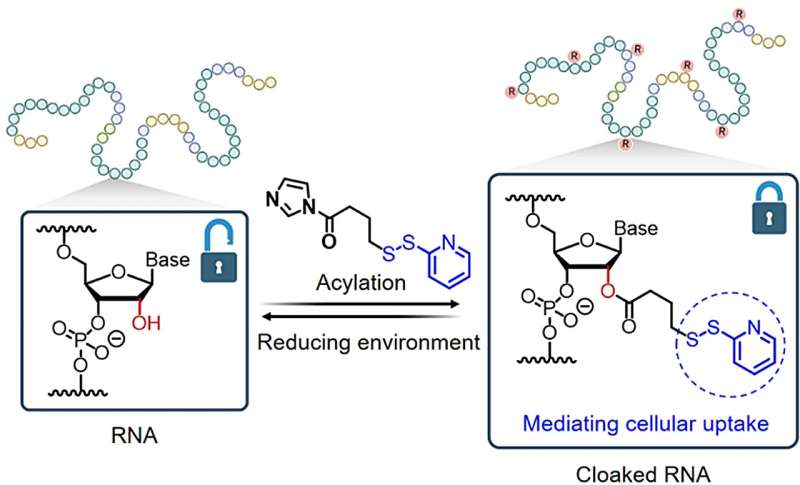
A research team led by Assistant Professor Zhu Ru-Yi, from the Department of Chemistry at NUS have developed a method that takes advantage of a chemical process called post-synthetic RNA acylation chemistry, and combined it with dynamic disulfide exchange reaction for RNA delivery and reversible control. The research findings are published in the journal Angewandte Chemie International Edition.
This method provides a way to mask the RNA molecule, and researchers can potentially regulate its activity and delivery until it reaches its target site within the cell.
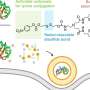
The researchers found that by adding special chemical markers (disulfide-containing groups) to the RNA, these groups can block RNA's catalytic activity and folding, temporarily hiding the instructions. Then, when needed, they can activate the RNA by removing these markers, allowing cells to read and act upon the instructions again.
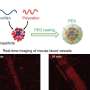
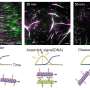
This strategy allows the RNA to enter cells quickly, distribute effectively, and become active in the cell's cytosol without getting trapped in lysosomes. The researchers believe that their methodology will be accessible to laboratories engaged in RNA biology and holds promise as a versatile platform for RNA-based applications.
Prof Zhu said, "Our studies showcase the first example of RNA delivery into cells using only small molecules."
"The simplicity of our method for modifying RNA and the unique delivery mechanism will undoubtedly attract more researchers to adopt and improve the method. We believe that our work will facilitate numerous applications in the field of RNA biology and biomedicines," added Prof Zhu.
Looking ahead, the research team is actively designing new strategies to modify RNA and improve RNA-based therapeutics.
More information: Junsong Guo et al, RNA Control via Redox‐Responsive Acylation, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202402178
Journal information: Angewandte Chemie International Edition
Provided by National University of Singapore