This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New study unveils unique roles of yeast protein complexes in cellular lifespan
Assistant Professor Takahiro Kosugi from the Institute for Molecular Science, assistant Professor Yoshiaki Kamada at the National Institute for Basic Biology, and colleagues have developed an advanced molecular cell biology approach by integrating computational redesigning of protein complexes based on the predicted three-dimensional structure in yeast genetics.
They revealed that two types of protein complexes in yeast, which were thought to have the same function, play distinct roles in cellular environmental response and lifespan. Furthermore, they showed that two complexes function differently in response to stress and they uncovered potential pathways for treating age-related diseases. The research is published in the Journal of Cell Science.
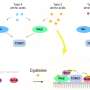
Proteins, serving as cellular workhorses, often form complex structures to execute essential functions. One such vital protein complex is TORC1, which plays a pivotal role in orchestrating cellular responses to environmental stimuli such as nutrient availability. This complex is not only linked to various diseases but also plays a significant role in lifespan regulation in a wide range of organisms, including humans.
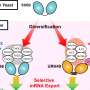
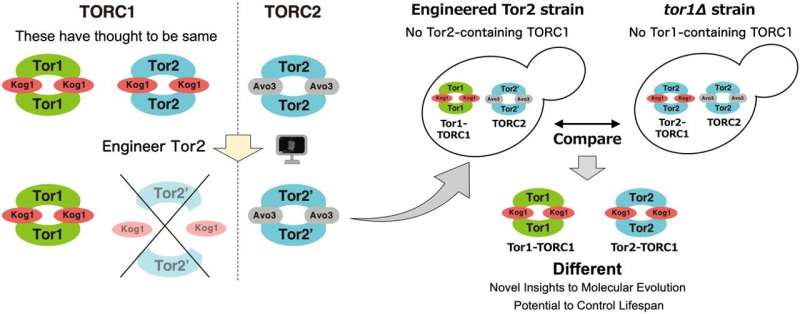
Unlike many organisms, one of the yeast species, Saccharomyces cerevisiae, possesses two different types of TORC1 complexes, each incorporating either the Tor1 or Tor2 proteins. This research challenges the previous assumption that these complexes are functionally redundant, merely existing as backups for each other. The research team shows the distinct and non-redundant roles of the two complexes.
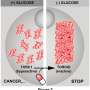
The research team embarked on a mission to uncover the differences between the two types of yeast TORC1 complexes. By engineering a mutant version of the Tor2 protein, they effectively prevented it from forming TORC1 complex while allowing it to maintain the ability to form TORC2.
This strategic alteration enabled the researchers to analyze the distinct functions of the two TORC1 complexes, particularly focusing on the one containing Tor2.
The study showed that yeast cells lacking Tor2-containing TORC1 responded differently to various environmental stresses compared to wild-type cells or cells lacking Tor1-containing TORC1. For instance, cells without Tor2-TORC1 exhibited higher sensitivity to TORC1 inhibitors including rapamycin and caffeine.
Additionally, the research team examined the effects of the TORC1 alteration on the yeast's lifespan. The findings were compelling: the absence of Tor2-containing TORC1 resulted in unique lifespan characteristics, distinctly different from those observed in cells without Tor1-containing TORC1.
These results provide new insights into the molecular evolution of Tor complexes, cellular signaling pathways, and lifespan regulation. The study not only sheds light on the specific functions of Tor1- and Tor2-containing TORC1 in yeast but also opens avenues for further exploration in the context of human biology and disease.
Their advanced approach of using three-dimensional structure-based engineering of target protein complexes to address a biological question is a noteworthy point. Three-dimensional structures of target proteins were computationally generated and then the mutants were selected based on a robust rationale rooted in these model structures.
Consequently, among a small number of candidates, one mutant was as designed. Also, the structures are model complex structures predicted computationally and not on the experimental structures. This approach has the potential to be a general method because it can be applied to an extensive array of high-quality predicted protein structure models, made available by recently developed high-accuracy structure prediction methods using deep learning. It may be possible to engineer native proteins based on their predicted structure and uncover their biological functions, as this study has done.
The findings of this study hold profound implications for advancing our understanding of cellular aging and disease treatment. By distinguishing the non-redundant functions of the TORC1 complex variants in yeast, the research team has laid the groundwork for future medical interventions that could target these pathways more precisely. This could revolutionize therapeutic strategies for a variety of age-related diseases and conditions linked to cellular nutrient response.
Furthermore, given the conservation of the TOR pathway through evolution, these yeast models could foreshadow similar breakthroughs in human health, potentially offering new avenues for treating complex diseases such as cancer, diabetes, and neurodegenerative disorders.
This study not only provides insight into the specific roles of TORC1 in yeast but also underscores the potential for predicted protein structure-based genetic engineering to unveil the intricate workings of cellular life, potentially enhancing human health and longevity.
More information: Yoshiaki Kamada et al, Structure-based engineering of Tor complexes reveals that two types of yeast TORC1 produce distinct phenotypes, Journal of Cell Science (2024). DOI: 10.1242/jcs.261625
Provided by National Institutes of Natural Sciences