This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Team successfully synthesizes atomically precise metal nanoclusters
A research team has successfully synthesized a metal nanocluster and determined its crystal structure. Their study provides experimental evidence for understanding and designing nanoclusters with specific properties at the atomic level. Metal nanoclusters have wide-ranging applications in the biomedical field.
Their work is published in the journal Polyoxometalates.
Scientists have shown interest in ligand-protected atomically precise metal nanoclusters because they have definite atomic structures and exceptional physical and chemical properties. These properties include attributes such as luminescence, chirality, electrochemistry, and catalysis.
Because of these properties, metal nanoclusters hold promise as ideal model catalysts. With their ultrasmall size, these nanoclusters demonstrate high catalytic activity and are selective in many catalytic reactions.
Ligand-protected metal nanoclusters are ultra-small organic–inorganic nanostructures that demonstrate high stability at specific compositions. Because of their tunable properties, they have the potential for a variety of nanotechnology-based applications.
Metal nanoclusters that share similar structures, but are made up of different metals, provide researchers with a unique opportunity for in-depth study of the atomic level metal synergy. To completely harness the potential of these metal nanoclusters in various applications, researchers need to be able to synthesize alloy nanoclusters with similar structures but distinct metal compositions.
This synthesis allows researchers to conduct a comprehensive examination of the factors influencing the nanoclusters' properties. Although researchers have made significant progress in preparing metal nanoclusters with similar structures, the availability of these nanoclusters is limited. The synthesis of similar metal nanoclusters is an essential next step for researchers.
Over time, research into these alloy nanoclusters has attracted increasing attention among researchers. From previous studies, researchers have gained a preliminary understanding of the origin of the optical properties of metal nanoclusters. With this, they can obtain theoretical guidance for designing nanoclusters with high photoluminescence quantum yields.
The research team undertook a study of the gold silver nanocluster [Au7Ag8(SPh)6 ((p-OMePh)3P)8]NO3 (Au7Ag8). They synthesized this nanocluster, analyzed its crystal structure, and examined its optical and electrocatalytic carbon dioxide reduction properties.
The team used single-crystal X-ray diffraction, electrospray ionization mass spectrometry, X-ray photoelectron spectroscopy, and thermogravimetric analysis to study the nanocluster. Their experimental results matched their theoretical calculations.
"Our work can aid in obtaining a better understanding of the effect of metal synergy on optical and catalytic properties at the atomic level," said Shuxin Wang, who is a professor at the College of Materials Science and Engineering at Qingdao University of Science and Technology in China.
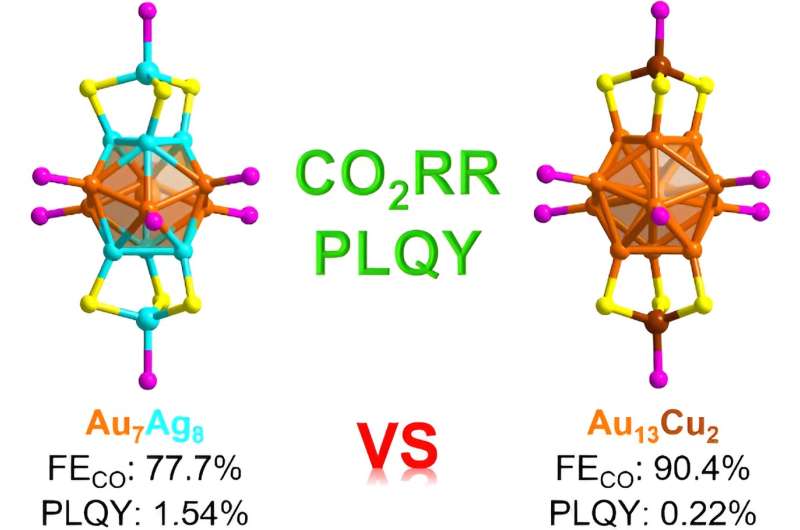
For comparison, they also synthesized a similar gold copper nanocluster, [Au13Cu2(TBBT)6((p-ClPh)3P)8]SbF6 (Au13Cu2). They compared the optical and electrocatalytic carbon dioxide reduction properties of the two metal nanoclusters. Both of these metal nanoclusters exhibit the same core structure, which is fundamentally identical, but they are different in their metallic compositions.
When they compared the optical and catalytic properties of the two nanoclusters, the Au7Ag8 had a photoluminescence quantum yield that was considerably greater than the photoluminescence quantum yield for the Au13Cu2.
They discovered that compared with copper doping, silver doping effectively enhanced the photoluminescence quantum yield of the nanocluster by a factor of 7. The two nanoclusters also exhibited different catalytic properties.
When they examined the electrocatalytic carbon dioxide reduction reaction, the addition of a small quantity of copper, while enhancing the catalytic selectivity for carbon monoxide production, concurrently reduces the electrochemically active surface area. From their structural analysis, the team attributes the superior carbon monoxide selectivity to the copper doping in the Au13Cu2 nanocluster.
In an ideal electrocatalyst, researchers would want to achieve a delicate balance between selectivity and preservation of an optimal electrochemically active surface area. Looking ahead, the team is working to incorporate multiple metals. "We hope to achieve synergistic catalysis for enhanced selectivity and efficiency," said Wang.
More information: Along Ma et al, Atomically precise M 15 (M = Au/Ag/Cu) alloy nanoclusters: Structural analysis, optical and electrocatalytic CO 2 reduction properties, Polyoxometalates (2024). DOI: 10.26599/POM.2024.9140054
Provided by Tsinghua University Press