This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Strain at monolayer MoS₂/hBN interfaces enhances hydrogen evolution reaction activity
Recently, the research team led by Prof. Wang Bin at National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences reported that strain generated at bubbles of 2D materials could benefit the catalytic activity of hydrogen evolution reaction (HER). The study was published in Chem Catalysis.
Green hydrogen produced by electrochemical water splitting offers the potential to achieve carbon-neutral production processes. Catalysts play a crucial role in facilitating HER at the anode, making it a key component in the transition to a sustainable energy future.
Transition metal dichalcogenides (TMDs), particularly MoS2, have drawn attention to replacing platinum-based materials. A series of strategies such as defect, doping, vacancy, and interface engineering have been implemented to improve the catalytic activity of the MoS2 basal plane for the HER.
However, the influence of out-of-plane microstructures (such as wrinkles or ripples, scrolls or folds, and bubbles) has often been overlooked, which commonly exist in 2D materials due to their flexibility. Therefore, the correlation between the active sites and the tested performance of catalysts is still questionable, especially considering the easy appearance of curved morphology in practical catalysts.
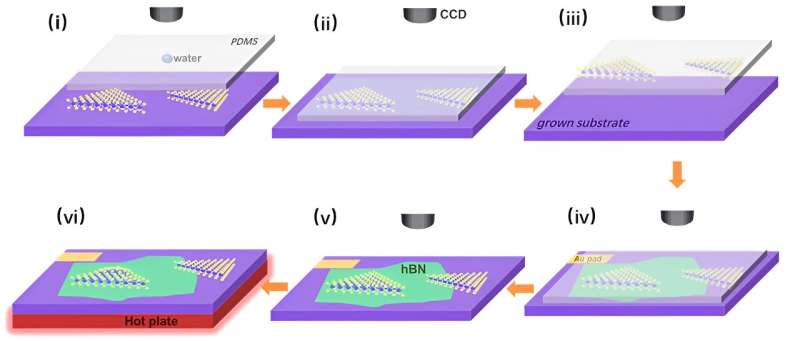
In this study, Prof. Wang's team, inspired by the bubbles that were fabricated via the top-down approach, realized the tailoring of bubbles with different "substrate-free" curvature at the interfaces between monolayer MoS2 and hBN by a droplet-assisted transfer method.
Finite element modeling (FEM) calculations demonstrated a gradual increase in strain distribution, moving from the bubble's periphery towards its center. Large bubbles can reach strain levels as high as 1.74%.
Density functional theory (DFT) showed that these bubbles induce strain formation on MoS2, which enhances the adsorption of protons and HER kinetics. Consequently, there was a substantial boost in HER activity, with values reaching 129.65 mA cm-2 compared to 48.11 mA cm-2 at -0.4 V vs. reversible hydrogen electrode (RHE).
"Our team has discovered an innovative method for fabricating bubbles, enabling precise customization and providing insights into the profound influence of bubbles on strain distribution. Experimental results showed that the strain level associated with larger bubbles surpasses the typical lattice distortion-induced strains.
"We believe that this finding has important implications for understanding the intricate relationship between out-of-plane structures and the intrinsic material properties," said Prof. Wang.
Besides, theoretical studies showed that the strain appeared in such out-of-plane structures could tune the electronic structure and thus adjust the proton adsorption performance of catalysts, which not only provides a more efficient and stable catalyst for hydrogen energy production but may also drive technological advances in other related fields.
More information: Junjie Xiong et al, Strain derived from bubbles at monolayer MoS2/hBN interfaces for enhanced hydrogen evolution reaction activity, Chem Catalysis (2024). DOI: 10.1016/j.checat.2024.100951. www.cell.com/chem-catalysis/ab … 2667-1093(24)00075-7
Provided by Chinese Academy of Sciences