This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists identify novel lysosome fission factor
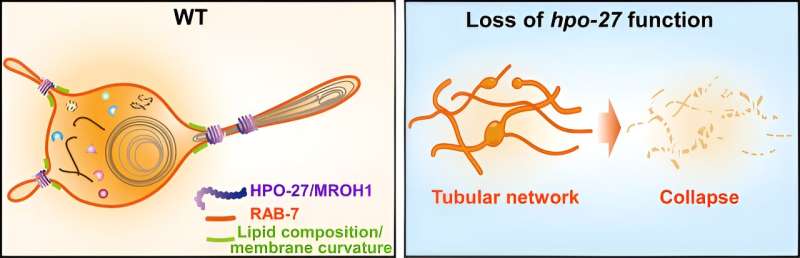
Lysosomes are centers for degradation, recycling, and signaling of cellular materials that are crucial for maintaining cellular homeostasis, development, and aging. To meet various physiological demands, lysosomes continuously remodel their shape and function through fusion and fission processes. While lysosome fusion process has been extensively studied, little is known about lysosome fission. The molecules responsible for lysosomal membrane fission have not been identified.
A research group led by Profs. Wang Xiaochen and Feng Wei from the Institute of Biophysics of the Chinese Academy of Sciences has discovered a new lysosomal membrane fission factor and elucidated its mechanism of action.
The study was published in Nature.
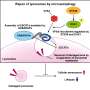
The researchers established a multicellular organism lysosome research system using Caenorhabditis elegans as a model and identified the HEAT repeat protein HPO-27, whose human homolog is MROH1, by forward genetic screening. HPO-27 and MROH1 contain 37 HEAT repeat sequences, and their functions are not yet understood.
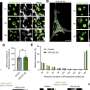
By combining live cell imaging and cryo-electron microscopy experiments, the researchers found that HPO-27 is widely expressed in multiple developmental stages and tissues of C. elegans. Functional analysis of lysosomes showed that defects in HPO-27 and MROH1 result in abnormal lysosomal acidification, reduced hydrolytic enzyme activity, and weakened degradative capacity.
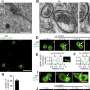
Co-localization analysis and continuous imaging with super-resolution microscopy revealed that HPO-27 and MROH1 are recruited to lysosomes by RAB7 and regulate lysosomal membrane fission.
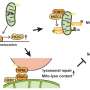
Through structural prediction, truncation body construction, super-resolution microscopy, negative-stain electron microscopy, and in vitro reconstitution, the researchers found that individual HPO-27 or MROH1 can oligomerize by head-to-tail binding and mediate the constriction and fission of lysosomes and reconstituted membrane tubes in C. elegans, mammalian cells, and in vitro systems.
This study has discovered a novel class of membrane fission factors that mediate membrane tube fission without direct consumption of energy molecules (ATP/GTP).
The discovery of HPO-27 and MROH1 provides a new avenue for studying lysosomal membrane fission and other membrane fission processes, laying an important foundation for further elucidating the regulation of lysosomal homeostasis and physiological functions.
More information: Letao Li et al, The HEAT repeat protein HPO-27 is a lysosome fission factor, Nature (2024). DOI: 10.1038/s41586-024-07249-8
Journal information: Nature
Provided by Chinese Academy of Sciences